RR3 How do changes in temperature affect the
advertisement

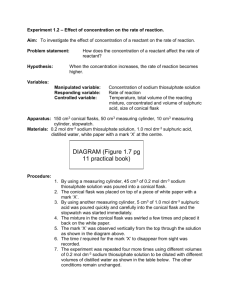
RR3 How do changes in temperature affect the speed of a chemical reaction ? IDEA The reaction between sodium thiosulphate and dilute hydrochloric acid will be used. In this reaction a precipitate of sulphur is formed slowly. The solution becomes milkier and milkier until you cannot see through it. WORD EQUATION sodium + hydrochloric thiosulphate acid sodium chloride (salt) + sulphur 2NaCl + S CHEMICAL EQUATION 2HCl Na2S2O3 + + + water H 2O + + sulphur dioxide SO2 The temperature of the sodium thiosulphate will be changed by heating the solution to a known temperature. The concentration of the acid remains unchanged. DIAGRAM PROCEDURE Put 50 mls of sodium thiosulphate in to the conical flask. Place this solution on a tripod and gauze and heat the solution for a few minutes. Draw a small cross on the piece of paper and put the flask on top of it. Pour the acid into the hot sodium thiosulphate solution and start the clock. Record the temperature. Time (in seconds) how long it takes for the cross to disappear. Wash out the flask thoroughly . Repeat using the same volumes of sodium thiosulphate and acid as given in the results table. Vary the temperature of the sodium thiosulphate THE RESULTS: Fill in the table below Volume of sodium thiosulphate (mls) 50 Volume of acid used (mls) 5 Temperature (oC) . Time (secs) . 50 5 . . 50 5 . . 50 5 . . 50 5 . . 50 5 . . Using the graph paper below plot a graph of temperature of sodium thiosulphate solution (vertical axis ) against the time taken for the cross to disappear (horizontal axis). QUESTIONS 1. At what temperature was the reaction at its (i) fastest ? (ii) slowest ? 2. How does the speed of reaction vary with temperature ? 3. Explain why a change in temperature can alter the speed of a reaction ? Talk about the reacting particles. FOR THE TECHNICIAN HAZARDS 50 ml measuring cylinder (plastic) Sulphur dioxide 10 ml measuring cylinder (glass) Produced in this reaction is test tube toxic by inhalation. bunsen burner Students suffering from tripod asthma need to take care. gauze Hydrochloric acid stopclock Corrosive thermometer -10 - 110 oC May also cause burns sheet of white paper 2M hydrochloric acid (DANGER) sodium thiosulphate containing 40 g / litre Also an irritant.