Lesson - Griffin Education
advertisement
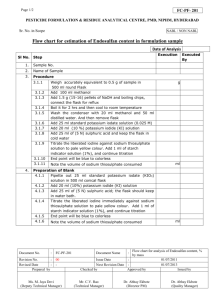
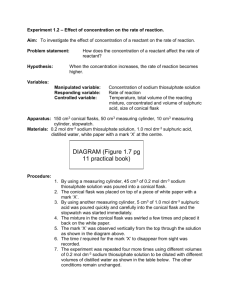
GRIFFIN EDUCATION working with EDU-LAB and The RADMASTE Centre UNIT C2 TOPIC 8.5 HOW FAST? HOW FURIOUS? Rate of reaction - the effect of concentration Teacher’s Notes Aims of sheet and practical The aims are for pupils to: • Follow a set of instructions. • Appreciate that the rate of a simple chemical reaction can be followed by appearance of products. • Plot a graph of the results. • Interpret these results. • Appreciate that the reaction is dependent on collisions between the particles involved. Apparatus and chemicals Comboplate© Hydrochloric acid (2 moldm-3). Sodium thiosulphate solution (80gdm-3). A white background with a coin or a cross against which the appearance of the sulphur is measured. A good idea here is to make a cross on a piece of white paper, photocopy it and laminate your copies. In this way you have a set of identical crosses and you can evaluate the performances of individual pupils. (* see below) Tap water. Clean micropipettes. SAFETY & RISK ASSESSMENT The hydrochloric acid is an irritant at this concentration and should be labelled as such. SAFETY SPECTACLES must be worn. If swallowed:- Wash out mouth and give a glass or two of water. Do not induce vomiting. Seek medical attention as soon as possible. If liquid gets in the eyes:- Flood the eye with gently running tap water for 10 minutes. Seek medical attention. If spilt on skin or clothes:- Flood affected area with large quantities of water. Remove contaminated clothing. If a large area is affected or blistering occurs, seek medical attention. NOTE The sulphur dioxide produced is toxic by inhalation and irritating to the eyes and respiratory system. Pupils with known breathing difficulties must not inhale the gas: IT MAY TRIGGER A HEART ATTACK When pouring the contents away make sure they do not inhale the sulphur dioxide fumes. They should be gently washed down the sink. Sodium thiosulphate solution has minimal hazards but would be harmful if ingested in quantity. Rate of reaction - the effect of concentration Student Worksheet 1. Using a micropipette add the sodium thiosulphate solution provided and water to wells A1 to A8 as shown in the table below:- 2. Using a pen or pencil draw a ‘X’ on some white paper. Place well A8 over the ‘X’ so that you can see the ‘X’ from above. 3. Using a clean propipette add 5 drops of hydrochloric acid provided and on the 5th drop start your stopwatch. 4. View the ‘X’ from above and stop your stopwatch the instant you can no longer see it. 5. Record the time in the table below. 6. Repeat steps 3 to 5 with wells A2 up to A8. Results Presentation of results Plot a graph of:Drops of sodium thiosulphate (horizontal or x-axis) against Time (sec) taken for ‘X’ to disappear (vertical or y-axis) Questions 1. The equation for the reaction is:- What happened when the acid was added to the sodium thiosulphate solution? _________________________________________________________________ Which of the products causes this to happen? Explain your answer. ___________ 2. How can this be used to tell you how fast the reaction is? 3. Therefore the shorter the time taken for the ‘X’ to disappear the _______________ is the reaction. 4. Which of the 8 wells has:a) the greatest concentration of sodium thiosulphate? ______________________ b) the lowest concentration of sodium thiosulphate? _______________________ 5. Using your results graph and answers to questions 3 and 4, state the effect on the reaction time of increasing the concentration of sodium thiosulphate. _________________________________________________________________ 6. Which of the 8 wells has :a) the fastest reaction? ______________________________________________ b) the slowest reaction? _____________________________________________ 7. What is the effect on the rate of reaction of increasing the concentration of sodium thiosulphate? 8. Complete the paragraph below using some of the words in the box. Note that some words are not to be used. Rate of reaction - the effect of concentration Assignment Introduction: You are asked to plan an experiment to find the effect of the concentration of sodium thiosulphate on the rate of this reaction. Prediction: Using collision theory predict the effect of increasing the concentration of sodium thiosulphate on reaction rate. _______________________________________________________________________ _______________________________________________________________________ _______________________________________________________________________ _______________________________________________________________________ _______________________________________________________________________ Make a numerical prediction on the same effect. Explain your reasoning. _______________________________________________________________________ _______________________________________________________________________ _______________________________________________________________________ _______________________________________________________________________ _______________________________________________________________________ _______________________________________________________________________ _______________________________________________________________________ Name THREE factors you will keep constant in order to make it a fair test. a. _____________________________________________________________________ b. _____________________________________________________________________ c. _____________________________________________________________________ Apparatus provided Chemicals provided 1 Comboplate© 3 Micropipettes Stopwatch Hydrochloric acid (CARE - this is corrosive) Sodium thiosulphate solution Trial run: Describe what happens when 5 drops of hydrochloric acid are added to 8 drops of sodium thiosulphate in well A1. _______________________________________________________________________ _______________________________________________________________________ _______________________________________________________________________ _______________________________________________________________________ _______________________________________________________________________ _______________________________________________________________________ _______________________________________________________________________ Results Conclusions What do your results show? _______________________________________________________________________ _______________________________________________________________________ Explain how you can tell this. ________________________________________________ _______________________________________________________________________ _______________________________________________________________________ Use the diagrams below to give a scientific explanation for your results. _______________________________________________________________________ _______________________________________________________________________ _______________________________________________________________________ _______________________________________________________________________ _______________________________________________________________________ _______________________________________________________________________ _______________________________________________________________________ _______________________________________________________________________ _______________________________________________________________________ _______________________________________________________________________ Is your numerical prediction correct? __________________________________________ Using your results explain your answer. _______________________________________________________________________ _______________________________________________________________________ _______________________________________________________________________ _______________________________________________________________________ Evaluation Give some good and bad points about your experiment. Good points:_______________________________________________________________________ _______________________________________________________________________ _______________________________________________________________________ _______________________________________________________________________ _______________________________________________________________________ _______________________________________________________________________ Bad points:_______________________________________________________________________ _______________________________________________________________________ _______________________________________________________________________ _______________________________________________________________________ _______________________________________________________________________ ____________________________________________________________