Reaction Rate Lab Sulfur Clock

Chemistry 122 Reaction Rate Lab Sulfur Clock 1
INTRODUCTION
In past discussions you have dealt with the rate of a chemical reaction in a qualitative (descriptive) manner. During the present laboratory exercise we will view the rate of a chemical reaction quantitatively. Rate is taken to mean a change per unit of time.
Some common examples:
Rate of travel - Kilometres/hour
Typing speed - 50 words per minute
Pulse rate - 65 beats per minute
For our purposes, the rate of a chemical reaction will be expressed as millimoles per second. The reaction is the decomposition of the thiosulphate ion in the presence of hydrogen ions.
S
2
O
3
-2 (aq) + 2H +1 (aq) ↔ S(s) + H
2
SO
3
(aq)
The solid sulfur produces a colloidal suspension, so that the solution becomes cloudy and opaque. The timing for the reaction begins with the mixing of the two solutions. The timing is stopped when a X mark under the beaker is no longer visible.
Reaction times will be recorded for various initial concentrations of the thiosulphate ion and different initial temperatures.
PROCEDURE
PART I - Varying the concentration
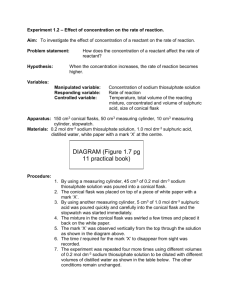
The first part of this experiment involves varying the initial concentration of the sodium thiosulphate solution while keeping the concentration of the hydrochloric acid and the temperature constant.
1. Place 50.0 ml of the 0.30M sodium thiosulphate solution into the beaker.
2. Set the beaker on the "X".
3. Obtain 5.0 ml of the 2.0M HC1 solution([H
+1
] = 2.0M).
When you are ready to start the reaction, pour the hydrochloric acid solution into the sodium thiosulphate solution. Gently swirl the beaker. Begin timing the reaction upon the addition of the acid solution . Stop the timing the moment the X is totally obscured from sight. Repeat the reaction to ensure consistent timings. (Your teacher will assign work groups.)
4. Repeat steps 1-3, reducing the concentration of the S
2
O
3
2-
ion by using 50.0 mL of:
0.25 mol/L Na
2
S
2
O
3
,
0.20 mol/L Na
2
S
2
O
3
,
0.15 mol/L Na
2
S
2
O
3
,
0.10 mol/L Na
2
S
2
O
3
and
0.05 mol/L Na
2
S
2
O
3
.
The quantity of 2.0 mol/L HCl added is always 5.0mL.
5. Continue in this manner, each time reducing the [S
2
O
3
2-
].
Chemistry 122 Reaction Rate Lab Sulfur Clock 2
PART II - Varying the temperature
The last part of this experiment involves the reaction being run at different temperatures. The concentrations of the solutions are constant. The sodium thiosulphate solution is 0.10M and the hydrochloric acid solution is 2.0M. The temperatures for the water baths are:
1. 10 ±2°C
2. 20 ±2°C
3. 30 ±2°C
4. 40 ±2°C
5. 50 ±2°C
Use the “ X” mark as indicated in Part I. Set up a water bath for your assigned temperature. Pour 50.0 ml of the 0.10M sodium thiosulphate solution into the marked beaker. Pour 5.0 ml of the 2.0M HCl solution into a test tube. Place the beaker and the test tube into the water bath. When the sodium thiosulphate solution has reached the assigned temperature, add the hydrochloric acid solution. Begin the timing with the mixing of the two solutions. The timing is to be stopped as soon as the X is no longer visible (while looking through the mixture of solutions). Repeat the reaction to assure consistency in your timing. (Your teacher will assign work groups.)
BEFORE leaving the laboratory collect class data.
MAKING SENSE OF THE DATA
Varying the concentration
1.
Experimentation has shown the amount of colloidal sulfur that must be produced to obscure the X is 0.00025 moles or 0.25 millimoles. In other words, all the timings represent the time required to produce 0.25 millimoles of sulfur. Calculate the rate of reaction for each mixture in Part I (Rate is expressed as millimoles of sulfur per second).
2.
Plot a graph of initial rate vs [S
2
O
3
-2
(aq)].
Varying the temperature
1) Calculate the rate of the reaction for each temperature in Part II. (Assume 0.25 millimoles of sulfur are produced.)
2) Plot a graph of rate vs temperature
3)
What effect on the rate is there for a change of 10°C?
Reaction Rate Lab Sulfur Clock Chemistry 122
X X X X
X X X X
X X X X
X X X X
3