Chemistry of Life
advertisement

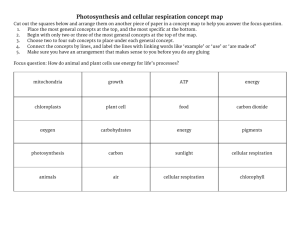
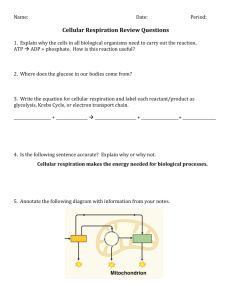
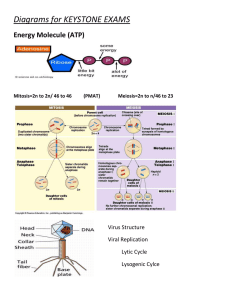
Chemistry of Life Key Elements • Element: composed of only one kind of atom; cannot be broken down to a simpler structure. • Six elements make up 99% of all living tissue – sulfur, phosphorus, oxygen, nitrogen, carbon, and hydrogen (SPONCH). • Carbon is most important because one carbon atom can make covalent bonds (e- are shared) with 4 other atoms; carbon is in ALL living things. • Organic: contains carbon; Inorganic: no carbon present (ex- water). Carbohydrates • Carbohydrates store energy. The starch in a potato is a good example. They also act as structural components, such as the cellulose that makes a celery stalk stringy or the chitin that composes the outer skeleton of a beetle. carbohydrates Lipids • Lipids perform a variety of functions. Fats store energy. Cell membranes are made of a type of structural lipid. Sex hormones, such as testosterone and estrogen, are made of lipids. The pigment chlorophyll, which is important in photosynthesis, is a lipid. lipids Proteins • Proteins are what the cell uses to translate the information in DNA into cell products. Proteins act as enzymes, which are important in making chemical reactions happen in cells. There are also structural proteins. Hair and cartilage are good examples. proteins Nucleic Acids • Nucleic acids include DNA, which carries genetic information, and RNA, which translates that information into proteins. • DNA (deoxyribonucleic acid) directs cell activities and contains the sugar deoxyribose. • RNA (ribonucleic acid) is involved in protein synthesis and contains the sugar ribose. nucleic acids Cell Composition • • • • • Water – 70% Proteins – 15% Fats – 10% DNA and other materials – 4% Carbohydrates – 1% Section 1 Review (p 71 – 72) • Answer numbers 2-4 in Multiple Choice. • Answer 1 – 3 in Short Answer. • Answer 1 – 3 in Fill in the Blanks. Cellular Respiration Energy and Chemical Bonds • Two types of bonds – ionic (electrons are lost or gained giving an atom a charge; opposite charges attract; metal and nonmetal); covalent (electrons are shared between atoms; nonmetal and nonmetal). • The stronger the bond, the more energy it contains. • When bonds are broken in the mitochondria, the energy they contain is released and becomes “free”, or useable, energy. • Of the energy used, about 60% is lost as heat, making respiration about 40% efficient. ATP (adenosine triphosphate) • Molecule that serves as chemical energy supply for all cells. • Made of adenine, ribose (sugar), and 3 phosphates. Covalent bonds between phosphate groups contain much energy. • After ATP breaks down, ADP is formed and combines with a free phosphate to form a new ATP molecule. Each ATP molecule is recycled this way 2000-3000 times per day. Obtaining Cellular Energy • Photosynthesis The process of converting CO2, H2O, and light energy into O2 and high energy sugar molecules. There are 2 basic stages: light-dependent and lightindependent reactions (the Calvin Cycle). Light-Dependent Reactions • Happens inside a chloroplast. • Plastids engage in photosynthesis and store the food produced. • Pigments absorb light. 6H20 + 6CO2 → C6H12O6 + 6O2 • The end products of light-dependent reactions are ATP, oxygen, and NADPH (NADP+ is an electron acceptor). • The ATP and NADPH will be used in the light-independent reactions, and the oxygen will be released into the atmosphere. Light-Independent Reactions • Also called carbon fixation reactions. • Uses the ATP formed in the lightdependent reactions as an energy source. • Carbon (from CO2) combines with NADPH to form glucose. • This glucose can be used as food to enter cellular respiration or can be converted to other carbohydrate products (sucrose or starch). Cellular Respiration • The process of breaking down food molecules to release energy. • There two types – aerobic and anaerobic. • Aerobic respiration requires oxygen to proceed; anaerobic happens without oxygen. • Energy released by cellular respiration is used to produce ATP. • The three phases of cellular respiration are glycolysis, Kreb’s cycle, & electron transport. C6H12O6 + 6O2 → 6CO2 + 6H20 + ENERGY • The respiration reaction is the opposite of photosynthesis. • Respiration occurs in the cells of ALL organisms. • Enzymes ARE NOT used. • The process starts with one molecule of glucose. Anaerobic Respiration • C6H12O6 → 2C2H5OH + 2CO2 + energy • Takes place WITHOUT oxygen present. • Also called FERMENTATION. • Produces ETHANOL (ethyl alcohol) and lactic acid. • CO2 released is why bread rises and beer has bubbles. Chemosynthesis • The process by which inorganic materials are broken down and energy released. • Only happens with bacteria that live around thermal vents on the ocean floor or near volcanic vents like at Yellowstone National Park. Comparing Photosynthesis, Respiration, and Chemosynthesis HAND-OUT For remainder of class… • READ pages 74 -76, Catalysts and Enzymes; Food Energy. • Take THOROUGH notes. • Chapter 3 REVIEW, pages 85 – 86. a. 1 – 18 Multiple Choice • TURN IN FOR CREDIT