Chemistry in the Community 1C Quest Study Guide
advertisement

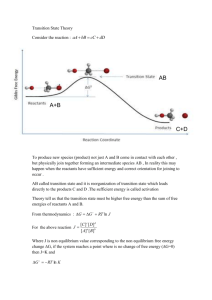
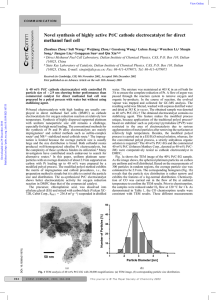
Chemistry in the Community 1C Quest Study Guide 1) Explain the mineral distribution on the Earth… are they evenly distributed or not evenly distributed? 2) Can metals be obtained profitably from low concentration (low-grade) ores? What makes them less desirable than high grade ores? 3) Name the process by which a metal is obtained from an ore. 4) Which “sphere” provides most of the raw materials needed by humans? 5) Know the most important factors needed to decide to mine a specific ore (there are 6 in the book) 6) Ca2+ + 2e- → Ca Is this an oxidation or reduction reaction? Be able to recognize AND write out either a reduction or oxidation reaction of a metal or ion in this form. 7) What are the resources found in each “sphere”? 8) To reduce a metal from an ore, you must use a metal (more or less) reactive than the metal you seek to extract from the ore. (Circle either more or less) 9) Write a balanced redox reaction for the oxidation of Pb metal to a Pb 2+ ion. Make sure you could do the same for a reduction reaction. 10) What are the percentages of the gases in the atmosphere? 11) OIL RIG 12) Write a balanced equation for the reduction of a zinc ion to a zinc atom (metal). Include the correct Lewis Structure (electron dots) 13) Why is mining copper fluoride (CuF) more profitable than mining copper sulfate (Cu(SO4)2)? 14) Using an activity series, how do you determine if a reaction will occur? 15) Magnesium (III) Nitrate ….Mg(NO3)3….contains how many atom of oxygen?______Why do you see a (III) written in the name? 16) Mg(s) + Ag+(aq) → Mg2+(aq) + 2Ag(s) ……which atom is oxidized? ______________which atom is reduced? ___________what is the oxidizing agent? __________what is the reducing agent?__________ 17) Which kinds of metals would be found uncombined in nature? 18) Name and describe 3 processes used to produce metals from metallic ores without having to use another metal. 19) What is the shortened term for an oxidation-reduction reaction? 20) Know the materials, purpose and general procedure of both labs (“Extracting zinc” and the video of “Copper plating”) completed in class. 21) Explain the usefulness of the mole concept in chemistry (think dozen) 22) Calculate the molar mass of a compound, given its formula and the atomic weights of its elements (WS) 23) Calculate the percent composition by mass of a specified element in a given compound (WS) 24) One mole of aluminum is how many grams? How many atoms? 25) List the diatomic elements 26) Describe how the electrical conductivity of a material can be changed (cathode and anode) 27) In the electroplating cell, the object to be plated should be the a)anode that is positive b) cathode that is positive c) anode that is negative, d) cathode that is negative 28) 4 moles of water is equal to how many molecules of water…show work to earn credit….ans= 2.408x1024 29) Which quantities contain the same number of atoms? A) 3 mol Ag and 3 mol N2 B) 1 mol CuCl 2 and 1 mol of ZnBr2 Why? 30) How many moles of Magnesium are found in a 6.0775-gram sample of magnesium (Mg)?