Chapter 1
advertisement

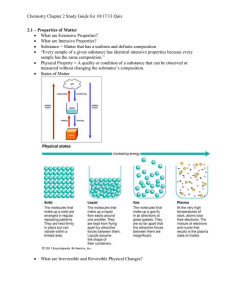
Chapter 1 Chemistry: The Science of Matter Fill in the blanks in your notes with the words bolded in orange. The Scientific Method The Scientific Method The scientific method is the systematic approach used in scientific study. Observation: is the act of gathering information by using your senses on a macroscopic level. Hypothesis: is a testable prediction used to explain an observation (if, then). Experiment: is a set of observations used to test a hypothesis. Control, independent variable, dependent variable Conclusion: addresses whether or not the hypothesis is supported by the results found The Scientific Method Theory: is an explanation based on many observations and supported by the results of many experiments. Scientific law: is a fact of nature that is observed so often that it is accepted as the truth. Benchmark If you haven’t done so already, you can now begin to do homework #1. Review slides 2-4 and your notes to help you answer these questions. You will not find the answers in your book. Matter & its Properties Chemistry is the science that investigates and explains the structure and properties of matter. Matter is anything that takes up space and has mass. Mass is the measure of the amount of matter that an object contains. The properties of matter describe the characteristics and behavior of matter, including the changes that matter undergoes. Matter & its Properties Physical Properties Characteristics that a sample of matter exhibits without any change in its identity. Example Matter & its Properties Chemical Properties The ability of a substance to combine with or change into one or more other substances. Example Matter & its Properties Matter & its Properties Macroscopic Matter that is large enough to be seen. Microscopic Matter that cannot be seen with the naked Matter & its Properties Qualitative An observation that can be made without measurement. Quantitative An observation that uses measurement. Benchmark If you haven’t done so already, you should read pages 3-15 in your textbook. With the reading, slides 6-11, and your notes should be able to complete homework #2. Classifications of Matter Mixtures & Physical Changes Heterogeneous Mixture A mixture with different compositions. Homogeneous Mixture A mixture that is the same throughout. It is also referred to as a solution. Mixtures & Physical Changes Mixtures & Physical Changes Types of Solutions… Solid Solutions: Alloys are solid solutions that contain different metals and sometimes nonmetallic substances. Liquid Solutions: The solute is the substance that is being dissolved. The solvent is the substance that does the dissolving. When the solvent is water, the solution is called an aqueous solution. Mixtures & Physical Changes A mixture is a combination of two or more substances in which the basic identity of each substance is not changed. Mixtures can be separated into its components by physical processes. Mixtures & Physical Changes Separation Techniques… Filtration Distillation Used to separate a liquid mixture. Crystallization Used to separate a mixture with widely varying particles size. Used to separate an aqueous solution. Chromatography Separates a mixture based on polarity. Benchmark If you haven’t done so already, read pages 18-31. With the reading, slides #13-18, and your notes, you should be able to complete homework #3. Mixtures & Physical Changes A physical change is a change in matter that does not involve a change in the identity of individual substances. Matter exists in one of three states (solid, liquid, or gas) depending on its temperature. Any change in state is a physical change. If a substance is described as being volatile, it becomes a gas easily at room temperature. Substances & Chemical Changes Compounds This type of pure substance can be broken down into simpler substances. It is a chemical combination of two or more different elements joined together in a fixed proportion. Elements This type of pure substance cannot be broken down into simpler substances. They are the simplest form of matter. Substances & Chemical Changes Substances & Chemical Changes The properties of a compound are different from the properties of the elements that compose the compound. A formula is a combination of the chemical symbols that show what elements make up a compound and the number of atoms of each element. A substance is matter with the same fixed composition and properties. Things that are pure are made up of only one kind of matter. Compounds can be separated into their component elements by chemical means. Substances & Chemical Changes A chemical change is a process that involves one or more substances changing into new substances. It is also referred to as a chemical reaction. The law of conservation of mass (matter) says that in a chemical change matter is neither created nor destroyed. Substances & Chemical Change Chemical changes involve an energy change. Energy is the capacity to do work. Work is done whenever something is moved. Chemical reactions that give off heat energy are called exothermic reactions. Chemical reactions that absorb heat energy are called endothermic reactions. Density Density is a physical property of matter. Density is the amount of matter (mass) contained in a unit of volume. The density of solids and liquids is usually measured in grams (mass) per milliliter (volume). g/ml For irregularly shaped objects, water displacement is used to obtain a volume measurement. Formula: Density Examples… What is the density of a piece of wood that has a mass of 25.0 grams and a volume of 29.4 cm3? I threw a plastic ball in the pool for my dog to fetch. The mass of the ball was 125 grams. What must the volume be to have a density of 0.500 g/ml? I want the ball to float, of course! Benchmark If you haven’t done so already, read pages 34-44. With the reading, slides #20-27, and your notes, you should be able to complete homework #4. Be proud of yourself, you just made it through your first unit in chemistry!