Chapter 1 Notes
advertisement

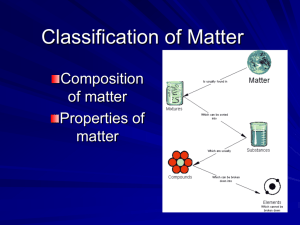
Chapter 1 Chemistry: The Science of Matter Fill in the blanks in your notes with the words bolded in orange. Warm Up Question List the senses that can be used to make an observation. Which one should definitely not use in a chemistry lab? 9/10 The Scientific Method The scientific method is the systematic approach used in scientific study. Observation: is the act of gathering information by using your senses on a macroscopic level. Hypothesis: is a testable prediction used to explain an observation (if, then). Experiment: is a set of observations used to test a hypothesis. Control, independent variable, dependent variable Conclusion: addresses whether or not the hypothesis is supported by the results The Scientific Method Theory vs. Law Theory: is an explanation based on many observations and supported by the results of many experiments. Scientific law: is a fact of nature that is observed so often that it is accepted as the truth. Explanation Arise following many, many experiments Statement Benchmark If you haven’t done so already, you can now Complete homework #1, part A. Review slides 3-5 and your notes to help you answer these questions. You will not find the answers in your book. Exit Question Read the following experiment: Gloria wanted to find out if the color of food would affect whether kindergarten children would select it for lunch. She put food coloring into 5 identical bowls of mashed potatoes. The colors were plain, red, green, yellow, and blue. Each child chose a scoop of potatoes of the color of their choice. What do you think the results would be? Write a hypothesis for this experiment using a “If…..then statement. Warm up Question 9/12 Write 3 words to describe this substance Matter & its Properties Chemistry is the science that investigates and explains the structure and properties of matter. Matter is anything that takes up space and has mass. Mass is the measure of the amount of matter that an object contains. Mass is not the same as weight. Weight is affected by gravity where mass is not. The properties of matter describe the characteristics and behavior of matter, including the changes that matter undergoes. Matter & its Properties Physical Properties Characteristics that a sample of matter exhibits without any change in its identity. Chemical Properties The ability of a substance to combine with or change into one or more other substances. Matter & its Properties Matter & its Properties Macroscopic Matter that is large enough to be seen. Microscopic Matter that cannot be seen with the naked Matter & its Properties Qualitative An observation that can be made without measurement. Quantitative An observation that uses measurement. Benchmark If you haven’t done so already, you should read pages 3-15 in your textbook. With the reading, slides 9-13, and your notes should be able to complete homework #1, part B. Exit Question Is color a physical or chemical property of matter? Explain 9/20 Warm up Question Both of these are mixtures. Explain how they are different from one another (besides their physical states) Classifications of Matter Mixtures A mixture is a combination of two or more substances in which the basic identity of each substance is not changed. ► Mixtures can be separated into its components by physical processes. Heterogeneous Mixture A mixture with different compositions. Homogeneous Mixture A mixture that is the same throughout. It is also referred to as a solution. Types of Solutions Solid Solutions: Alloys are solid solutions that contain different metals and sometimes nonmetallic substances. Liquid Solutions: The solute is the substance that is being dissolved. The solvent is the substance that does the dissolving. When the solvent is water, the solution is called an aqueous solution. Separation Techniques Filtration Distillation Used to separate a liquid mixture. Crystallization Used to separate a mixture with widely varying particles size. Used to separate an aqueous solution. Chromatography Separates a mixture based on polarity. Benchmark If you haven’t done so already, read pages 18-31. With the reading, slides #17-20, and your notes, you should be able to complete homework #2, part A. Exit Question What kind of mixture does this represent? How could you separate it into its individual components? Warm up Question 9/14 Name the separation technique is used to make coffee or tea? Physical Change A physical change is a change in matter that does not involve a change in the identity of individual substances. Matter exists in one of three states (solid, liquid, or gas) depending on its temperature. Any change in state is a physical change. If a substance is described as being volatile, it becomes a gas easily at room temperature. Mixtures are separated by a physical change Substances Compounds This type of pure substance can be broken down into simpler substances. It is a chemical combination of two or more different elements joined together in a fixed proportion. Elements This type of pure substance cannot be broken down into simpler substances. They are the simplest form of matter. Compounds The properties of a compound are different from the properties of the elements that compose the compound. A formula is a combination of the chemical symbols that show what elements make up a compound and the number of atoms of each element. A substance is matter with the same fixed composition and properties. Things that are pure are made up of only one kind of matter. Compounds are separated into their component elements by chemical means. Chemical Change A chemical change is a process that involves one or more substances changing into new substances. It is also referred to as a chemical reaction. The law of conservation of mass (matter) says that in a chemical change matter is neither created nor destroyed. C25H52 + 38 O2 --> 25 CO2 + 26 H2O Energy Change Chemical changes involve an energy change. Energy is the capacity to do work. Work is done whenever something is moved. Chemical reactions that give off heat energy are called exothermic reactions. Chemical reactions that absorb heat energy are called endothermic reactions. Benchmark If you haven’t done so already, read pages 18-31. With the reading, slides #24-28, and your notes, you should be able to complete homework #2, Part B. Exit Question List which of the following represent a physical change? Warm-up Question 9/24 Assuming that both of these objects take up the same space, state which object is more dense. Explain Wood Steel Density Density is a physical property of matter. Density is the amount of matter (mass) contained in a unit of volume. The density of solids and liquids is usually measured in grams (mass) per milliliter (volume). g/ml For irregularly shaped objects, water displacement is used to obtain a volume measurement. Formula: Density Examples… What is the density of a piece of wood that has a mass of 25.0 grams and a volume of 29.4 cm3? I threw a plastic ball in the pool for my dog to fetch. The mass of the ball was 125 grams. What must the volume be to have a density of 0.500 g/ml? I want the ball to float, of course! Benchmark If you haven’t done so already, read pages 34-44. With the reading, slides #32-34, and your notes, you should be able to complete homework #5. Be proud of yourself, you just made it through your first unit in chemistry! Exit Question List which of the following represent a chemical change?