Rates of reactions - jedlik.phy.bme.hu!
advertisement

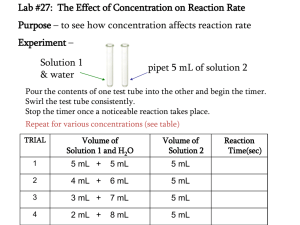
Rates of Reactions Tímea Szentgyörgyi SEK Budapest International School 1021 Hungary, Budapest, Hűvösvölgyi u. 131. Tel.:36-1-394-2968; Fax: 36-1-200-6615 www.sek.hu; www.sek.net Chemical kinetics is the study of the speed with which a chemical reaction occurs and the factors that affect this speed. The rate of a reaction is the speed at which a reaction happens. All the molecular level of life some reactions go very rapidly and other take forever. The rate of reaction, r, is defined to be the slope of the concentration-time plot for a species divided by the stoichiometric coefficient of that species. A+2B=3C r = -d[A]/dt = -1/2 d[B]/dt= 1/3 d[C]/dt The reactants have a negative slope, because they are being consumed in the reaction. Products have a positive slope, because they are being formed in the reaction. What factors affect the rate of a reaction? The concentration of the reactants. The more concentrated the faster the rate. Remember for gasses, increasing the pressure simply increases the concentration so that's the same thing. What factors affect the rate of a reaction? Temperature. Usually reactions speed up with increasing temperature. Physical state of reactants. Powders react faster than blocks greater surface area and since the reaction occurs at the surface we get a faster rate. The presence of a catalyst (or inhibitor). A catalyst speeds up a reaction, an inhibitor slows it down. Reaction with High Rates: 1. FeCl3 + KSCN → Fe( SCN)3 + 3 KCl 2. Faro’s snake Reaction with Middle Rates: FeCl3 + Na2S2O3 → [ Fe( S2O3)2 ]- Halloween reaction / Old Nassau Reaction The reaction in this experiment takes place in several steps [5]. First, sodium metabisulphite reacts with water to form sodium hydrogen sulphite: Na2S2 O5 + H2O ==> 2NaHSO3 Hydrogen sulphite ions reduce iodate(V) ions to iodide ions: IO3- + 3 HSO3-==> I- + 3 SO42+3H+ Once the concentration of iodide ions is large enough that the solubility product of HgI2 is exceeded, orange mercury(II) iodide solid is precipitated until all of the Hg2+ ions are used up (provided that there is an excess of Iions). Hg2+ + 2 I- ==> HgI2 (orange or yellow) If there are still I- and IO3 - ions in the mixture, the iodide-iodate reaction IO3-+5 I- + 6 H+ ==> 3 I2 + 3 H 2O takes place and the blue starch-iodine complex is formed, I2 +starch ==>complex (blue or black) Reactions with low rates: 1. Pb( CH3COO)2 + 2KI = PbI2 +CH3COOK 2.NiCO3+2 dmg= 3.CoSO4+2NH4SCN=Co(SCN)2 + [Ni( dmg)2]2+ + CO32(NH4)2 SO4 Thank you for your attention