Reaction Rate vs. Concentration: Iodine Clock Experiment
advertisement
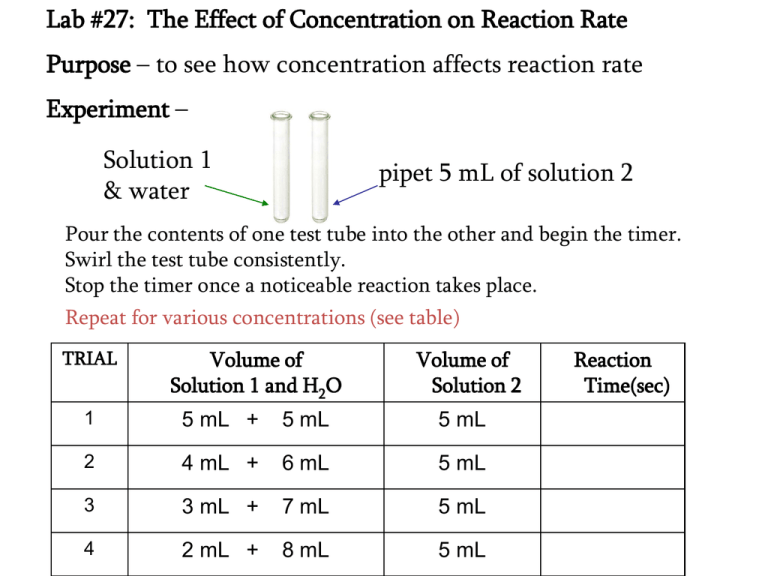
Lab #27: The Effect of Concentration on Reaction Rate Purpose – to see how concentration affects reaction rate Experiment – Solution 1 & water pipet 5 mL of solution 2 Pour the contents of one test tube into the other and begin the timer. Swirl the test tube consistently. Stop the timer once a noticeable reaction takes place. Repeat for various concentrations (see table) TRIAL Volume of Solution 1 and H2O Volume of Solution 2 1 5 mL + 5 mL 5 mL 2 4 mL + 6 mL 5 mL 3 3 mL + 7 mL 5 mL 4 2 mL + 8 mL 5 mL Reaction Time(sec) Analysis: • Properly graph your data Results: Write a statement that includes how reaction rate is effected by the concentrations…make sure to include actual data to support your statement. How the Iodine Clock Reaction Works (no notes!) STEP 1: Rate-determining step 3HSO3- + IO3sol. 1 + sol. 2 3H2O 3SO4-2 water + I- 3H3O+ + colorless ions STEP 2: IO3- + 5I- + sol. 2 6H3O+ 3I2 + dark blue 9H2O STEP 3: I2 + dark blue HSO3sol. 1 + 4H2O 2I- + SO4-2 + 3H3O+ colorless ions As long as there are HSO3- ions in the test tube that can react with the I2, no visible color change will occur.