Chapter Eleven
advertisement

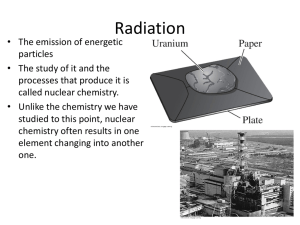
Fundamentals of General, Organic, and Biological Chemistry 5th Edition Chapter Eleven Nuclear Chemistry James E Mayhugh Oklahoma City University 2007 Prentice Hall, Inc. Outline ► 11.1 Nuclear Reactions ► 11.2 The Discovery and Nature of Radioactivity ► 11.3 Stable and Unstable Isotopes ► 11.4 Nuclear Decay ► 11.5 Radioactive Half-Life ► 11.6 Radioactive Decay Series ► 11.7 Ionizing Radiation ► 11.8 Detecting Radiation ► 11.9 Measuring Radiation ► 11.10 Artificial Transmutation ► 11.11 Nuclear Fission and Nuclear Fusion Prentice Hall © 2007 Chapter Eleven 2 11.1 Nuclear Reactions ► The atomic number, written below and to the left of the element symbol, gives the number of protons in the nucleus and identifies the element. ► The mass number, written above and to the left of the element symbol, gives the total number of nucleons, a general term for both protons (p) and neutrons (n). ► The most common isotope of carbon, for example, has 12 nucleons: 6 protons and 6 neutrons: Prentice Hall © 2007 Chapter Eleven 3 ► Nuclear reaction: A reaction that changes an atomic nucleus, usually causing the change of one element into another. A chemical reaction never changes the nucleus. ► Different isotopes of an element have essentially the same behavior in chemical reactions but often have completely different behavior in nuclear reactions. ► The rate of a nuclear reaction is unaffected by a change in temperature or pressure (within the range found on Earth) or by the addition of a catalyst. ► The nuclear reaction of an atom is essentially the same whether it is in a chemical compound or in an uncombined, elemental form. ► The energy change accompanying a nuclear reaction can be up to several million times greater than that accompanying a chemical reaction. Prentice Hall © 2007 Chapter Eleven 4 11.2 The Discovery and Nature of Radioactivity ► In 1896, the French physicist Henri Becquerel noticed a uranium-containing mineral exposed a photographic plate that had been wrapped in paper. ► Marie and Pierre Curie investigated this new phenomenon, which they termed radioactivity: The spontaneous emission of radiation from a nucleus. ► Ernest Rutherford established that there were at least two types of radiation, which he named alpha and beta. Shortly thereafter, a third type of radiation was found and named for the third Greek letter, gamma. Prentice Hall © 2007 Chapter Eleven 5 When passed between two charged plates: ► Alpha rays, helium nuclei (He+2), bend toward the negative plate because they have a positive charge. ► Beta rays, electrons (e-), bend toward the positive plate because they have a negative charge. ► Gamma rays, photons (g), do not bend toward either plate because they have no charge. Prentice Hall © 2007 Chapter Eleven 6 ►Alpha rays move about ~0.1c and can be stopped by a few sheets of paper or by the top layer of skin. ►Beta rays move at up to 0.9c and have about 100 times the penetrating power of a particles. A block of wood or heavy clothing is necessary to stop b rays. ►Gamma rays move at c and have about 1000 times the penetrating power of a rays. A lead block several inches thick is needed to stop g rays. Prentice Hall © 2007 Chapter Eleven 7 11.3 Stable and Unstable Isotopes ►Every element in the periodic table has at least one radioactive isotope, or radioisotope, and more than 3300 radioisotopes are known. ►Their radioactivity is the result of having unstable nuclei, although the exact causes of this instability are not fully understood. Radiation is emitted when an unstable radioactive nucleus, or radionuclide, spontaneously changes into a more stable one. ►There are only 264 stable isotopes among all the elements. ►All isotopes of elements with atomic numbers higher than that of bismuth (83) are radioactive. Prentice Hall © 2007 Chapter Eleven 8 ►For elements in the first few rows of the periodic table, stability is associated with a roughly equal number of neutrons and protons. ►As elements get heavier, the number of neutrons relative to protons in stable nuclei increases. ►Lead-208, for example, the most abundant stable isotope of lead, has 126 neutrons and 82 protons in its nuclei. Prentice Hall © 2007 Chapter Eleven 9 11.4 Nuclear Decay ► Nuclear decay: The spontaneous emission of a particle from an unstable nucleus. ► Transmutation: The change of one element into another. ► The equation for a nuclear reaction is not balanced in the usual chemical sense because the kinds of atoms are not the same on both sides of the arrow. A nuclear equation is balanced when the number of nucleons and the sums of the charges are the same on both sides. Prentice Hall © 2007 Chapter Eleven 10 ► During alpha emission, the nucleus loses two protons and two neutrons. ► Emission of an a particle from an atom of uranium-238 produces an atom of thorium-234. Prentice Hall © 2007 Chapter Eleven 11 ► Beta emission involves the decomposition of a neutron to yield an electron and a proton. ► Iodine-131, a radioisotope used in detecting thyroid problems, undergoes nuclear decay by b emission to yield xenon-131. Prentice Hall © 2007 Chapter Eleven 12 ► Positron emission involves the conversion of a proton in the nucleus into a neutron plus an ejected positron. ► A positron has the same mass as an electron but a positive charge. ► Potassium-40 undergoes positron emission to yield argon-40. Prentice Hall © 2007 Chapter Eleven 13 ► Electron capture, symbolized E.C., is a process in which the nucleus captures an inner-shell electron from the surrounding electron cloud, thereby converting a proton into a neutron. ► The conversion of mercury- 197 into gold-197 is an example of electron capture. Prentice Hall © 2007 Chapter Eleven 14 ► Emission of g rays causes no change in mass or atomic number. ► g emission usually accompanies emission of other rays but it is often omitted from nuclear equations. ► Their penetrating power makes them both dangerous to humans and useful in medical applications. Prentice Hall © 2007 Chapter Eleven 15 11.5 Radioactive Half-Life ► Rates of nuclear decay are measured in units of halflife, defined as the amount of time required for onehalf of the radioactive sample to decay. ► Each passage of a half-life causes the decay of one half of whatever sample remains. The half-life is the same no matter what the size of the sample, the temperature, or any other external conditions. Prentice Hall © 2007 Chapter Eleven 16 All nuclear decays follow the same curve, 50% of the sample remains after one half-life, 25% after two half-lives, 12.5% after three half-lives, and so on. Prentice Hall © 2007 Chapter Eleven 17 Radioisotopes used internally for medical applications have short half-lives so that they decay rapidly and do not remain in the body for prolonged periods. Prentice Hall © 2007 Chapter Eleven 18 11.6 Radioactive Decay Series ►Decay series: A series of nuclear disintegrations leading from a heavy radioisotope to a nonradioactive product. ►Uranium-238, for example, undergoes a series of 14 sequential nuclear reactions, ultimately stopping at lead-206. Prentice Hall © 2007 Chapter Eleven 19 11.7 Ionizing Radiation ► A large dose of ionizing radiation can destroy living cells, causing death. ► A small dose of ionizing radiation may not cause visible symptoms but might lead to a genetic mutation or cancer. Prentice Hall © 2007 Chapter Eleven 20 ► Health professionals who work with X rays or other kinds of ionizing radiation protect themselves by surrounding the source with a thick layer of lead or other dense material. ► Protection is also afforded by controlling the distance between the worker and the radiation source because radiation intensity (I) decreases with the square of the distance from the source. ► The intensities (I) of radiation at two different distances (d) are given by the equation: I1d12 = I2d22 Prentice Hall © 2007 Chapter Eleven 21 11.8 Detecting Radiation ►We cannot see, hear, smell, touch, or taste radiation, no matter how high the dose. We can, however, detect radiation by taking advantage of its ionizing properties. ►The simplest device for detecting exposure to radiation is the photographic film badge. ►The film is protected from exposure to light, but any other radiation striking the badge causes the film to fog. Prentice Hall © 2007 Chapter Eleven 22 ►The Geiger counter is an argon-filled tube. The inner walls are given a negative charge, and a wire in the center is given a positive charge. ►Radiation ionizes the argon atoms, which briefly conduct a current between the walls and the center electrode. ►The current is detected, amplified, and used to produce a clicking sound or to register on a meter. Prentice Hall © 2007 Chapter Eleven 23 ► The most versatile method for measuring radiation in the laboratory is the scintillation counter. ► In this device, a substance called a phosphor emits a flash of light when struck by radiation. ► The number of flashes are counted electronically and converted into an electrical signal. Prentice Hall © 2007 Chapter Eleven 24 11.9 Measuring Radiation Some radiation intensity units measure the number of nuclear decay events; others measure exposure to radiation or the biological consequences of radiation. Prentice Hall © 2007 Chapter Eleven 25 ► The biological consequences of different radiation doses are shown below. ► The average annual radiation dose is only about 0.27 rem. About 80% of this background radiation comes from natural sources; the remaining 20% comes from medical procedures and from consumer products. Prentice Hall © 2007 Chapter Eleven 26 11.10 Artificial Transmutation ► Very few of the approximately 3300 known radioisotopes occur naturally. Most are made from stable isotopes by artificial transmutation, the change of one atom into another brought about by nuclear bombardment reactions. ► Carbon-14 is created in the upper atmosphere. 14N + 1n 14C + 1H ► Plutonium-239 is made in breeder reactors. 238U + 1n 239U 239Np + b- 239Pu + bPrentice Hall © 2007 Chapter Eleven 27 11.11 Nuclear Fission and Nuclear Fusion ► Nuclear fission: The fragmenting of heavy nuclei. ► Chain reaction: A reaction that is self-sustaining. ► Critical mass: The minimum amount of radioactive material needed to sustain a nuclear chain reaction. ► The fission of 235U produces vast amounts of heat that can be used to produce electric power. ► Lithuania and France generate about 86% and 77%, respectively, of their electricity in nuclear plants. About 22% of U.S. electricity is nuclear-generated. Prentice Hall © 2007 Chapter Eleven 28 Each fission event produces additional neutrons that induce more fissions. Such chain reactions usually lead to the formation of many different fission products. Prentice Hall © 2007 Chapter Eleven 29 ► Nuclear fusion: The joining together of light nuclei. ► Light nuclei such as the isotopes of hydrogen release enormous amounts of energy when they undergo fusion. It is fusion reactions of hydrogen nuclei to produce helium that powers our sun and other stars. ► The necessary conditions for nuclear fusion are not easily created on Earth. ► In stars the temperature is on the order of 2 x 107 K and pressures approach 1 x 105 atmospheres. At these extremes, nuclei are stripped of all their electrons and have enough kinetic energy that nuclear fusion readily occurs. Prentice Hall © 2007 Chapter Eleven 30 Chapter Summary ► A nuclear reaction changes an atomic nucleus, causing the change of one element into another. ► A nuclear reaction is balanced when the sum of the nucleons is the same on both sides of the reaction arrow and when the sum of the charges on the nuclei plus any ejected subatomic particles is the same. ► Radioactivity is the spontaneous emission of radiation from the nucleus of an unstable atom. a radiation consists of helium nuclei, b radiation consists of electrons, and g radiation consists of high-energy light waves. ► One half-life is the amount of time necessary for one-half of the radioactive sample to decay. Prentice Hall © 2007 Chapter Eleven 31 Chapter Summary Cont. ► High-energy radiation of all types is called ionizing radiation. When ionizing radiation strikes an atom, it makes a reactive ion that can be lethal to living cells. ► g rays and X rays are the most penetrating and most harmful types of external radiation; a and b particles are the most dangerous types of internal radiation. ► The curie (Ci) measures disintegrations per second; the roentgen (R) measures the ionizing ability; the rad measures the amount of radiation energy absorbed per gram of tissue; and the rem measures the amount of tissue damage caused by radiation. ► Radiation effects become noticeable with a human exposure of 25 rem and become lethal at an exposure above 600 rem. Prentice Hall © 2007 Chapter Eleven 32 Chapter Summary Cont. ►Transmutation is the change of one element into another brought about by a nuclear reaction. Most known radioisotopes do not occur naturally but are made by bombardment of an atom with a high-energy particle. ►In fission, the nucleus is split apart to give smaller fragments. A large amount of energy is released during fission, leading to its use for generating electric power. ►Nuclear fusion results when small nuclei such as those of tritium and deuterium combine to give a heavier nucleus. Prentice Hall © 2007 Chapter Eleven 33 End of Chapter 11 Prentice Hall © 2007 Chapter Eleven 34