CHAPTER 1 NOTES
advertisement

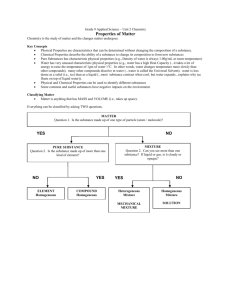
Chemistry is the study about how matter is put together, how atoms combine to form molecules, and how the molecules combine to make up the many kinds of matter around us. Classification of Matter Matter – identified and organized by properties anything that has mass and takes up space Properties Chemical – ability to react/combine (both what it will and won’t react with) - will change the composition Physical – characteristics observed or measured -will not change the composition two kinds of physical properties help describe the behavior of a substance undergoing a physical change 1. Extensive- depends on the amount of matter Ex: mass, length, volume 2. Intensive- does not depend on the amount of Ex: density (regardless of size, each sample of a substance has the same density) color, crystalline shape, melting point, boiling point, phase of the matter (solid, liquid, gas), and refractive index (ability of material to bend light) Property Physical or Chemical Intensive or Extensive Size P E Density P I Color P I Flammable C N/A Corrosive C N/A Phase of matter P I State (or phase) of matter is always a physical property: Solid, liquid, gas Phase Energy – move Particle spacing Volume Shape Compressible Solid Little Definite Definite No Liquid Some Close Rigid Close Slide Definite Not Definite No Gas Lot Far Not Definite Not Definite Yes Changes in Properties Physical change - does NOT change the composition (identity) of the substance. Same substance remains after a change has taken place *Size: pounding, bending, grinding, pulling, or cutting does not change the chemical character of a substance. *Change of state: melting, boiling, vaporizing *Cutting a piece of wood into smaller pieces, tearing paper, dissolving sugar in water, hammering copper into a new shape are all examples of physical change. Chemical changeDOES change composition. After a change has taken place a new substance appears Changes that produce a new kind of matter with new, different properties. Burning, digestion, fermenting all form new substances with new and different characteristics. Sodium- a silvery, soft metal that reacts vigorously with water (Na) Chlorine- a yellow-green gas that is highlycorrosive and poisonous (Cl) Yet if these two elements are brought together, they combine to form a white crystalline solid. Table salt (NaCl), which neither reacts with water, nor is poisonous. 1. Heat and/or light is produced 2. Production of a gas (bubbles) 3. Odor 4. Color change 5. Formation of a precipitate – a cloudy solid which appears after mixing to clear solutions Chemical Reactions - a Chemical change has taken place: 2 or more substances have chemically bonded together to create a new substance Law of Conservation of Mass – matter is neither created nor destroyed. This states that mass remains constant during a chemical reaction. One of the greatest scientific achievements of the 18th century by a Frenchman, Lavoisier (1743-1794) Mass (of reactants) = Before Mass (of products) After Chemical Equations: Reactant + reactant product + product (always on left) (always on right) ~ arrow shows directions Ex. 12 g of calcium reacts with 6 grams of fluorine gas to produce calcium fluoride. How many grams of product will be produced? 12 g Ca + 6 g F2 ____ g CaF2 (12 + 6) = (18) Try it Separating water turns into hydrogen gas and oxygen gas. If 10.0 g of hydrogen and 79.4 g of oxygen are produced, how much water did was there at the start? ____ g H2O 10.0 g H2 + 79.4 g O2 89.4 g H2O 10.0 g H2 + 79.4 g O2 Mixture- matter that contains two or more different materials Phase- any region with a uniform set of properties Ex: sour milk, watery part is one phase while fat is the second phase Mixtures are divided into two categories 1. Heterogeneous mixture- a mixture that is composed of more than one phase Ex: granite, sand 2. Homogeneous mixturematerials that consist of only one phase. If you break a piece of homogeneous matter into smaller pieces, each piece will have the same properties as every other small piece Ex: seawater, window glass, and air Separating Mixtures (physical separation) 1. Filtration – through a porous barrier, filters solids from liquids 2. Distillation – different boiling points 2 liquids: lowest boiling point vaporizes 1st 3. Crystallization – dissolved solids from a solutions rock candy left after water evaporates 4. Chromatography – different rate of travel through a medium identify chemicals, DNA testing, separates pigments, research Pure Substances- homogeneous materials that always have the same composition *Homogeneous mixtures are not pure substances, yet the substances that comprise them are evenly dispersed throughout the mixture Pure Substances are divided into two categories 2. Compounds 1. Elementssubstances composed of only one kind of atom (elements on the P.T.) Ex: sodium ~ Na, Iron ~ Fe, Neon ~ Ne composed of more than one kind of atom (made from multiple elements on the P.T.) Ex: water ~ H2O, table salt ~ NaCl Compounds cannot be physically separated, they are chemically bonded Matter 1. 2. Mixtures Homogeneous Mixture Pure Substances Heterogeneous Mixture Suspension Element 1. 2. Solutions 1. 2. 3. Colloid1. Compound 1. 2. 3. 2. Matter 1. Has Mass 2. Has Volume(Occupies Space) Mixtures Homogeneous Mixture Pure Substances Heterogeneous Mixture Suspension Element 1. 2. Solutions 1. 2. 3. Colloid1. Compound 1. 2. 3. 2. Mixtures Variable Composition Can be physically separated Homogeneous Mixture Heterogeneous Mixture Uniform Throughout (But Proportions can Vary) Solutions 1. Particles are atoms, ions, or molecules 2. Won’t scatter light 3. Ex. Salt Water, Steel, Air Not uniform Throughout Ex. Lumpy ColloidDon’t settle out 1. 2. 3. Particles larger than molecule Scatters light (Tyndall Effect) Ex. Smoke, Fog Suspension Solid in Liquid Eventually settle out Ex. Muddy water Wood, granite, blood Pure Substances Definite Composition Homogeneous Can’t be physically separated Element Compound 1. 2. Chemical combinations of 2 or more atoms. (elements chemically bonded) Cannot by separated by physical means (CaCl2, NaCl, Sugar, C6H12O6) 1. 2. Made of atoms Cannot be further separated by chemical or physical means (on periodic table Mendeleev arranged in rows-periods Columnsgroups/families) Matter 1. Has Mass 2. Has Volume(Occupies Space) Mixtures Pure Substances Variable Composition Can be physically separated Homogeneous Mixture Uniform Throughout (But Proportions can Vary) Definite Composition Homogeneous Can’t be physically separated Heterogeneous Mixture Not uniform Throughout Ex. Lumpy Solutions 1. Particles are atoms, ions, or molecules 2. Won’t scatter light 3. Ex. Salt Water, Steel, Air Colloid- Don’t settle out 1. 2. 3. Particles larger than molecule Scatters light (Tyndall Effect) Ex. Smoke, Fog Suspension Element Solid in Liquid 1. 2. Eventually settle out Ex. Muddy water Wood, granite, blood Compound 1. 2. Chemical combinations of 2 or more atoms. (elements chemically bonded) Cannot by separated by physical means (CaCl2, NaCl, Sugar, C6H12O6) Made of atoms Cannot be further separated by chemical or physical means (on periodic table Mendeleev arranged in rows-periods Columnsgroups/families) Law of Definite Proportions Regardless of amount, a compound is always composed of the same elements in the same proportion by mass % by Mass = Mass of element x 100 Mass of compound If compounds have the same % by mass, they must be the same 80 g sample of compound contains 20 g of hydrogen. What is the percent by mass of hydrogen? 20 g x 100 = 25% 80 g If 7 g of iron combine with 3 g of oxygen, what is the % by mass of iron? Of oxygen? Hint: Find the total mass 1st % iron = 7g x 100 = 70% 10 g %oxygen = 3g x 100 = 30% 10g Try it Sample 1 contains 15.0 g of H2 and 120.0 g of O2 Sample 2 contains 2.0 g of H2 and 32.0 g of O2 Are they the same compound? Sample 1 contains 15.0 g of H2 and 120.0 g of O2 Sample 2 contains 2.0 g of H2 and 32.0 g of O2 Are they the same compound? Sample 1 15 g + 120 g = 135 g Sample 2 2 g + 32 g = 34 g H2 : 15 x 100 = 11% 135 O2 : 120 x 100 = 89% 135 H2 : 2 x 100 = 6% 34 O2 : 32 x 100 = 94% 34 Different percentages so these are different compounds Intro to the Periodic Table Vertical columns Horizontal rows called groups or families. called periods. Elements in a group have similar chemical & physical properties. Numbered from 1-18 from left to right Elements within a period have properties that change progressively across the table. Metals – (left side) are good conductors of heat and electricity; shiny metallic luster (mostly silver or grayish white); malleable (hammered or rolled into thin sheets); ductile (string into a wire); properties vary according to groups Nonmetals – (right side) elements that are poor conductors of heat and electricity, dull (no luster); some are gases at room temperature. Br is a liquid; C, P, Se, S & I are solid and much more brittle than metals Metalloids – (on the stair-step line) – elements that have characteristics of both metals and nonmetals to a limited degree. Less malleable than metals, but not as brittle as nonmetals; some have luster, some don’t; semiconductors of electricity (used in semi-conducting materials found in computers, calculators, watches, TV’s and radios) Branches of Chemistry Organic Chem – study of carbon – containing compounds Inorganic Chem – all substances not classified as organic (mainly those that do not contain carbon) Physical Chem – the study of properties and changes of matter and their relation to energy Analytical Chem – identification of the components and composition of materials Biochemistry – substances and processes occurring in living things Theoretical Chem – uses math and computers to understand the principles behind observed chemical behavior and to design and predict new compounds