Key to Review Worksheet for Exam 1 (Ch. 11-Ch. 12.4)
advertisement

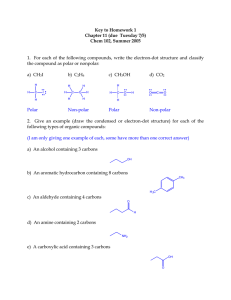
Key to Review Worksheet for Exam 1 (Ch. 11-Ch. 12.4) Chem 102, Fall 2005 1. For each of the following compounds, write the electron-dot structure (show all nonbonded electron pairs) and classify the compound as polar or nonpolar: a) CH3SH b) CH3CH2OH H H C S H H H Polar H H C C H H O c) C2H2 H H Polar C C H Nonpolar 2. Classify each of the following as an organic or inorganic compound: a) H2SO4 b) CH2CO2H c) CO2 In organic Organic Organic 3. Identify each of the following compounds by functional group: OH O a) b) O c) d) H 3C Alcohol Ether Ketone O O H N e) g) f) H Aldehyde CH Alkyne O h) NH2 OH Carboxylic Acid C Amine Amide 4. Give an example (draw the structure) for each of the following types of organic compounds: a) An alkene containing 3 carbons b) An ester containing 4 carbons O O c) An aromatic hydrocarbon containing 7 carbons CH3 d) An alcohol containing 2 carbons OH e) An ether containing 2 carbons O f) An amide containing 2 carbons O H N H g) A carboxylic acid containing 2 carbons O OH 5. Draw the structures for all of the constitutional isomers for each of the following molecular formulas: a) C4H8 (alkenes) b) C4H10O (alcohols) OH OH OH OH c) C4H8O2 (esters) O O O O O H O O H 6. Classify each of the carbons in the molecule below as 1, 2, 3 or 4: 1 3 2 2 3 2 4 1 1 1 O 7. Give a name for each of the following structures: Br a) b) 2,2-dimethylpropane 2-bromo-3-methylpentane F c) d) Cl 3-chloro-4-fluorohexane 2,3-dimethylpentane e) f) 4-isopropylheptane 3,3-dibromo-5-propyloctane Cl Br Cl g) h) Cl 2,2,3-trichlorobutane 4-ethyl-5-methylnonane 8. Draw the structure for each of the following names: a) 2,3-dibromobutane Br Br Br b) 4-ethyl-3-methylheptane c) 1,1,2,2-tetrachloroethane Cl Cl Cl Cl d) 4-isopropyloctane e) 3,6-dimethyl-5-propylnonane f) 2,3,3-trimethylpentane g) 3,4-diethylhexane