Lewis Structures and Bonding Basics
advertisement

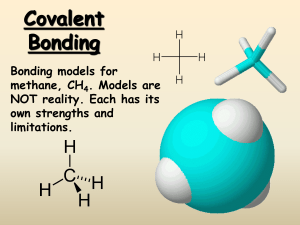
CHEMICAL BONDING & NAMING Before we begin… Exceptions to the octet rule: Atom # of e- to be satisfied Hydrogen (H) 2 Helium (He) 2 Lithium (Li) 2 Beryllium (Be) 4 Boron (B) 6 YOU MUST MEMORIZE THESE! Chemical Bonds The force that holds two atoms together is called a chemical bond usually accompanied by a change in energy. Chemical bonds form because of attractions between oppositely charged atoms, called ions, or between electrons and nuclei. Types of Chemical Bonds Valence electrons distribute themselves among ions to form chemical bonds Ionic bonds – the transfer of electrons due to an electrical attraction between a cation and an anion (usually a metal & a non-metal) Covalent Bond – the sharing of electrons between two atoms (usually 2 or more non-metals) Metallic Bond – bonding between metals How to determine type of bond The difference in electronegativity of the bonding atoms can determine the type of bond that forms 0.0 < ∆EN < 0.5 non polar covalent 0.5 ≤ ∆EN < 1.9 polar covalent ∆EN ≥ 2.0 ionic Percent Ionic Character Polarity Non – polar covalent - covalent bond that equally shares the electron (CO2) Polar covalent the electron is not equally shared (H2O) The charge distribution is represented with a δ (lower case delta) Showing Polarity +δ +δ H H O H H O -δ Lewis Structures Helps determine the shape of the molecule Dots represent electrons Lines represent bonds (need two electrons to form a bond) Rules Calculate the maximum number of valence electrons for each atom in the molecule (e- wanted) Remember exceptions (H, He, Li, Be, & B) Calculate the actual number of valence electrons for each atom in the molecule (e- have) Subtract e- have from e- wanted = shared eDivide shared e- in half = bonds formed Central atom is typically the atom in the center of the molecule or the atom which is single & least electronegative F2 SO42- More Practice