Review: Mini Test % comp, empirical, and molecular Name: Period
advertisement
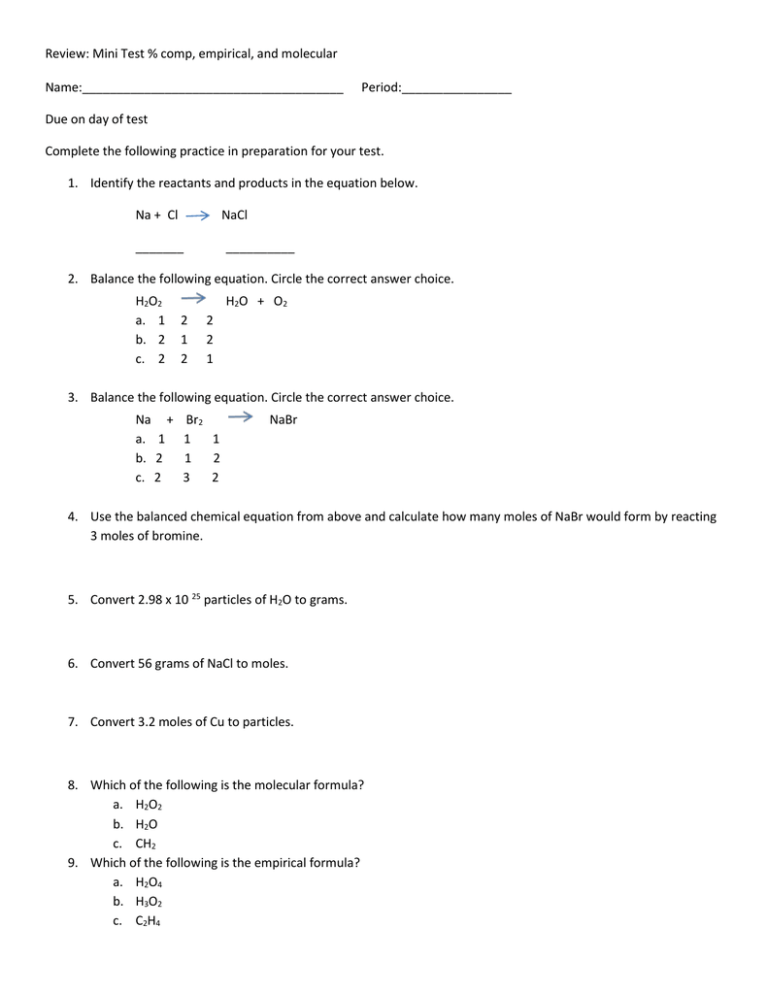
Review: Mini Test % comp, empirical, and molecular Name:______________________________________ Period:________________ Due on day of test Complete the following practice in preparation for your test. 1. Identify the reactants and products in the equation below. Na + Cl NaCl _______ __________ 2. Balance the following equation. Circle the correct answer choice. H2O2 a. 1 b. 2 c. 2 H2O + O2 2 1 2 2 2 1 3. Balance the following equation. Circle the correct answer choice. Na + a. 1 b. 2 c. 2 Br2 1 1 1 2 3 2 NaBr 4. Use the balanced chemical equation from above and calculate how many moles of NaBr would form by reacting 3 moles of bromine. 5. Convert 2.98 x 10 25 particles of H2O to grams. 6. Convert 56 grams of NaCl to moles. 7. Convert 3.2 moles of Cu to particles. 8. Which of the following is the molecular formula? a. H2O2 b. H2O c. CH2 9. Which of the following is the empirical formula? a. H2O4 b. H3O2 c. C2H4 Review: Mini Test % comp, empirical, and molecular 10. Find the percent composition of each element in the compound. (NH4)2S N: ___________ H: ___________ S: ___________ 11. Find the percent composition of each element in the compound. N2S2 N: ___________ S: ___________ 12. The percentage composition of acetic acid is found to be 39.9% C, 6.7% H, and 53.4% O. Determine the empirical formula of acetic acid. 13. The molar mass for question #9 was determined by experiment to be 60.0 g/mol. What is the molecular formula? 14. Vitamin C contains three elements: carbon, hydrogen, and oxygen. Analysis of pure vitamin C indicates that the elements are present in the following mass percentages: C = 40.9%, H = 4.58%, and O = 54.5%. Use the data to determine the empirical formula for vitamin C. 15. Using the empirical formula found in question 13, determine the molecular formula. Experimental data indicates that the molecular mass of vitamin C is about 180g/mol.