File - miss marsh science
advertisement

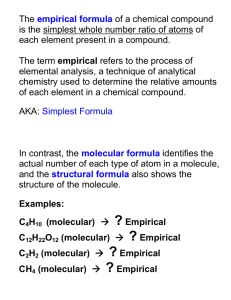
Empirical + Molecular formula formula 25/01/2015 Calculate empirical formula from experimental data. Quick recap. Molecular and empirical formula. Molecular formula is the actual ratio of atoms (only applies to covalent molecules) Empirical formula is the simplest ratio of atoms (always given for ionic compounds) Recap But what does it mean ? Copper oxide could be CuO or Cu2O 1. What is the Ar of Cu ? 2. What is the Ar of O ? 3. So if 64g of copper reacted with 16g of oxygen what would the empirical formula of the compound be? 4. If 128g of copper reacted with 16g of oxygen what would the empirical formula be ? What if I get horrible numbers? 13.6g of copper combined with 1.7g of oxygen what is the formula of the copper oxide ? Copper oxide could be CuO atoms Moles =Mass ÷ Ar Ratio (divide through by lowest denominator) or Cu2O Cu O What if I get percentages? A sample of a hydrocarbon was found Copper oxide could be CuO atoms Moles =Mass ÷ Ar Ratio (divide through by lowest denominator) or Cu2O Cu O What is I get percentage data ? e.g. A compound was found to consist of 85.7 % C and 14.3% hydrogen by mass. a. What is its empirical formula ? -just turn % directly into g. just pretend you were given 100g, (e.g. Whatever % you are given use it as grams). atoms Moles =Mass ÷ Ar Ratio (divide through by lowest denominator) C H What is I get percentage data ? Sometimes you might get data in percentage composition. -just pretend you were given 100g, (e.g. Whatever % you are given use it as grams). Example. a molecule contained 40% carbon, 6.7% hydrogen and 53% oxygen, what is its empirical formula ? atoms Moles =Mass ÷ Ar Ratio (divide through by lowest denominator) C H O What if I get experimental data for the question 1. Don’t freak out 1. Copper oxide was strongly heated in a boiling tube. The results are given below. a. Calculate the empirical formula of the copper oxide. Mass of empty tube 52.5g Mass of tube and copper 103.7g Mass of tube and copper oxide after heating 110.1g Find out the masses of each element. 103.7g- 52.5g = 51.2g 51.2g ÷ 64= 0.8 Cu= O= 110.1g-103.7g- = 6.4g 6.4g ÷ 16= 0.4 atoms Moles =Mass ÷ Ar Ratio (divide through by lowest denominator) Cu O atoms Moles =Mass ÷ RFM Ratio (divide through by lowest denominator) Mass of empty tube 40.5g Mass of tube and zinc oxide 81g Mass of tube and copper after 73g The other thing you might be asked is to go from empirical formula to molecular formula. e.g. A compound was found to have 85.7 % C and 14.3% hydrogen by mass. a. What is its empirical formula ? CH2 a. It has a relative formula mass of 56, what is its molecular formula ? How many times does the empirical formula “fit into” the molecular formula. The other thing you might be asked is to go from empirical formula to molecular formula. How many times does the empirical formula “fit into” the molecular formula. Empirical formula= CH2O = (12+2+16)=30 Molecular formula= C6H12O6 = 180 CH2O 180 = 6. 30 The other thing you might be asked is to go from empirical formula to molecular formula. e.g. A compound was found to have 85.7 % C and 14.3% hydrogen by mass. a. What is its empirical formula ? CH2 a. It has a relative formula mass of 56, what is its molecular formula ? e.g. A compound was found to have 85.7 % C and 14.3% hydrogen by mass. a. What is its empirical formula ? CH2 a. It has a relative formula mass of 56, what is its molecular formula ? CH2 C4H8 1. Work out empirical formula mass =(12)+ (2x1) = 14 2. Divide molecular formula mass By empirical formula mass =56g ÷ 14g=4 2. Multiply each atom ratio by this number 4 Now try worksheet Now try question on page 87, 89 and worksheet