Lh6Ch04bProt
advertisement
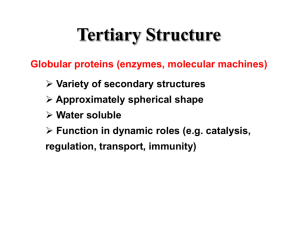
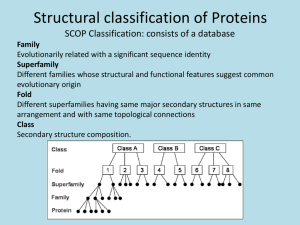
Chapter 4 The Three-Dimensional Structure of Proteins Part 2 Chapter 4, Part 2: Learning Goals 1. Know the structures and functions of collagens, role of ascorbic acid (vitamin C) in collagen structure. 2. Know globular protein structure and families. 3. Know how de-naturation and re-naturation works or sometimes doesn’t. Collagen Triple Helix Left Handed, 3 aa/turn Prolyl-4-hyroxylase Hydroxylates Protein as Procollagen Hyrdoxproline is necessary to keep some prolines in the “exo” form to allow the collagen triple helix to form. Prolyl-4-hydroxylase is a Di-oxygenase can Catalyze Two Reactions Without Vitamin C, the iron of the first enzyme becomes oxidized and Inactive. Ascorbate actually keeps the enzyme iron reduced although this diagram does not show it. Hydroxylysine Cross Links Collagen Triple Helix Strands Iriquois showing Jacques Cartier how to make Cedar Tea - a source of Vitamin C James Lind’s experiment could not be done today. Why? Did he lack a control group? Was there something else? Newly Discovered Bond in Collagen IV The Sulfilimine Bond Between a hydroxylysine and methionine Vanacore, R, et al. 2009. A sulfilimine bond identified in collagen IV. Science. 325:13230. Sept 4, 2009 Sulfilimine Bond – Evolutionary Conservation Human Serum Albumin (Mr = 64,500) if it was: This figure has a flaw. Horizontal dimensions are OK, Verticals are off in two ways: different scale and globular form is way too small. Structures of Myoglobin What about “random coil” or “random structure”? Where is it in myoglobin? - go back to previous slide, it represents 22% of the amino acids in myoglobin! Is it random? Yes and No!! Both are correct why? Is it coil? Yes and No!! Both are correct why? Heme in Myoglobin Structures of some Small Proteins A Troponin has 2 Domains Each Domain has a Distinct Function: Binding Ca++ Two Small Motifs Here alpha helix connects two beta-structures Alpha turn alpha are common on some DNA binding proteins Smaller Motifs into Large Motifs Protein Families – Classes and Folds All Beta Protein Families Alpha/Beta Protein Families Alpha + Beta Protein Families Max Perutz and John Kendrew Quaternary Structure of Hemoglobin 2 α and 2 β Quaternary Structure: Symmetry Polio Virus and Tobacco Mosaic Virus A Protein Stability and Folding • A protein’s function depends on its 3D-structure • Loss of structural integrity with accompanying loss of activity is called denaturation • Proteins can be denatured by: • heat or cold • pH extremes • organic solvents • chaotropic agents: urea and guanidinium hydrochloride Thermal and Chemical Protein Denaturation Irreversible Reversible or Urea Ribonuclease Refolding Experiment • Ribonuclease is a small protein that contains 8 cysteines linked via four disulfide bonds • Urea in the presence of 2-mercaptoethanol fully denatures ribonuclease • When urea and 2-mercaptoethanol are removed, the protein spontaneously refolds, and the correct disulfide bonds are reformed • The sequence alone determines the native conformation • Quite “simple” experiment, but so important it earned Chris Anfinsen the 1972 Chemistry Nobel Prize Reversible Unfolding with Mercaptoethanol CH3-CH2-SH This step must be done very slowly Simulated Folding Proteins folding follow a distinct path Creutzfledt-Jakob Disease: Human Spongiform Encephalopathy Vacuoles Contain a Missfolded Protein – in Brain Tissue Prions Infectious proteins Inherited and transmissible by ingestion, transplant, & surgical instruments PrPC, normal cellular prion protein, on nerve cell surface PrPSc, scrapie protein, accumulate in brain cells forming plaques Prion Miss-folding PrPSc PrPc 1 2 3 4 Lysosome Endosome 5 6 7 8 PrP Folding Chaperones prevent misfolding Chaperonins facilitate folding GroEL and GroES Protein Folding Alzheimer’s Disease, Type 2 Diabetes and Parkinson’s Disease A Amyloid Fibers Stabilized by F Different Amyloid diseases depend on organ the fibers occur A A Summary of Forces Driving Protein Structure 1. hydrophobic interactions contribute strongly to protein folding and stabilization ultimately burring hydrophobic R groups with at least two layers of secondary structure covering them up to exclude water. 2. alpha and beta structures are usually in different layers. Their R-groups generally do not allow mixing. 3. Secondary structure near each other (in primary sequence) are usually stacked (except in quaternary structure). 4. beta structure is most stable when slightly twisted. The great example being the beta-barrel (Fig 4-20) of many membrane proteins. 5. Beta bends can not form knots. Things to Know and Do Before Class 1. Know collagen structure and the role of vitamin C. 2. Structure of globular proteins, circular dichroism, and the main idea of protein families (there are over 800). 3. Denaturation and Renaturation (or not) of proteins 4. One of the largest unsolved puzzles in modern biochemistry: the details of how proteins fold. 5. Roles of Chaparones. 6. Be able to do EOC Problems 7-11 Problem 12 makes you calculate the molecular weight of the DNP-aa in the diagram.