File
advertisement

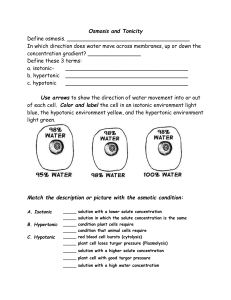
Unit: Cells Topic: Movement through the Cell Membrane Do Now- Using a Venn Diagram compare diffusion and osmosis. Answer examples: osmosis is a type of diffusion but requires semi-permeable membrane and water; both are passive transport no energy required Aim- What makes osmosis occur? Answer: Solute levels in water; water moves from low solute to high solute area. Standards S4 Students will understand and apply scientific concepts, principles, and theories pertaining to the physical setting and living environment and recognize the historical development of ideas in science. Performance Indicator : 1.2g Each cell is covered by a membrane that performs a number of important functions for the cell. These include: separation from its outside environment, controlling which molecules enter and leave the cell, and recognition of chemical signals. The processes of diffusion and active transport are important in the movement of materials in and out of cells. 1.2h Many organic and inorganic substances dissolved in cells allow necessary chemical reactions to take place in order to maintain life. Large organic food molecules such as proteins and starches must initially be broken down (digested to amino acids and simple sugars respectively), in order to enter cells. Once nutrients enter a cell, the cell will use them as building blocks in the synthesis of compounds necessary for life. Performance Objectives 1. Students will be able to describe what happens during osmosis. 2. Students will be able to analyze data from a grape activity. 3. Students will be able to compare and contrast how different solutions affect the cell. 4. Students will be able to distinguish between diffusion and osmosis. Vocabulary: Concentration- Concentration= the amount of substance in a particular area; Mass of solute in a given volume of solution= M/V Hypertonic- the concentration of solute outside the cell is greater than inside Hypotonic- the concentration of solute outside the cell is lower than inside the cell Isotonic- the concentration is the same on both sides (inside and outside the cell) Material List Grapes, cups, water, grape juice, corn syrup, napkins. Safety and Disposal Teacher will collect grapes. Students will throw out any used napkins. Anticipatory Opening Cartoon Image of Osmosis Jones. Activity: Students will be given a table data of the results from a previous activity involving grapes in 3 different solutions. One set of data was collected by them, and the other three by me at different times. They will analyze the data to determine the effects of the solutions on the grapes: hypertonic, hypotonic, and isotonic. They will also make observations of the grapes to see the effects. They will make rough graphs of the data and answer some questions. Development of Lesson: Follows LESSON ACTIVITY SEQUENCE: Estimated time: 5 min Teacher Activity(s): What will teacher do? Write Do Now, Aim and HW on the board. Venn Diagram: diffusion/osmosis comparison Set up material. Go over Do Now. Ask for volunteers (or pick on 2 students) to answer question. 1 min Anticipatory event. Image on board. 5 min Introduce lesson, show video and images, ask students questions. List important vocabulary words on the board:.Only provide definition of concentration at this point. (Review) Concentration- Concentration= the amount of substance in a particular area; Mass of solute in a given volume of solution= M/V Hypertonic- the concentration of solute outside the cell is greater than inside Hypotonic- the concentration of solute outside the cell is lower than inside the cell Isotonic- the concentration is the same on both sides (inside and outside the cell) Student Activity(s): What will students do? Start Do Now. Independent thinking of question and completing Venn Diagram. Answer questions, 2 students go to the board and illustrate and fill in diagram. Students get motivated. Students answer questions and take notes on vocabulary. Students get into groups and work on activity and questions. Prepare to answer questions and put graph on board. Students answer questions. 9 min Prepares students for activity. Gives instructions. Puts students in groups. Asks students questions, puts data on board. Passes out worksheet and procedure. Review with students their observations and ask students for definitions of hypertonic, hypotonic and isotonic. Have them provide definitions and put up your definitions on the board. 3 min Read the Aim. Guide students to use vocabulary. Student reads Aim. Answers Aim. 4 min Extension of lesson; ask students relevant questions. Students answer questions. 1 min Homework announcement. Students read HW. Summative assessment Ask questions. Students answer. 5 min Differentiated Instruction A few students participate more than others so there would be questions throughout the lesson to fill their need. There are also some students that are fairly quiet and will be randomly chosen to answer a question. Other students that do not generally participate will possibly be chosen through a system of drawing sticks with their names on it to answer questions. Students that like to come to the board will have a couple chances for this. Students that are more artistic have an opportunity to draw the graph. Recall, analysis, evaluating, and creating are skills they will be using at different points in the lesson. Collaboration and individual work is applied. The types of learners targeted here include, visual, logical/mathematical, artistic, linguistic, interpersonal and intrapersonal. Students will take notes, students will participate in interactive activities on the board. Notes for Revision: Homework Illustrate hypotonic, hypertonic, and isotonic effects on cells (plant or animal). Label and use coloring utensils. Describe what happens for each in a few sentences. OR: Do a Venn Diagram for Hypertonic, Hypotonic and Isotonic Summative Assessment -Students will be asked several questions related to the lesson: What happened to the paramecium in the video when salt was added to its environment? - It lost water, shriveled and died. What is an example of a hypertonic solution or situation? - More solutes than solvent; more sugar or salt in the water outside the membrane. How do red blood cells look when salt is added to their external environment? -Shriveled up Why did they look like that? What is that process called? -They look like that because the solution they were in was hypotonic, and the cells lost water. How is osmosis different from diffusion? -Osmosis is different from diffusion because it involves a semi-permeable membrane that requires water; moves down a solute concentration gradient (from low solute to high solute). When might you not want osmosis to occur? Explain. -When you want the cell to maintain its shape and water; when you want fruits to stay juicy and firm; when you want plants to not wilt; when you don’t want pesticides to diffuse in with the water. What is the importance of the concentration of a solution? -It will determine if the cell maintains the proper amount of water inside; it can determine if the cell (or organism, or fruit), shrivels, dies, wilts. Name: Group originally in A, B, or C (circle) Date: Osmosis Grapes? Worksheet to be completed by every student for collection. Procedure: 1. In your group of 4-5 people you will each be responsible for a task (analyze data, compare data, obtain the grapes for observations, and graph the data). 2. In your group you will discuss and analyze the results in the groups you were in last week (A,B, and C) from the data table included. 3. In your group you will also discuss and analyze the results from the other Groups and compare and contrast them to your Groups’ results. 4. Prepare a (rough) graph to illustrate the results of your group. Be prepared to present the results on the board and to speak about them. Questions: 1. What was the initial mass(g) and the final mass(g) of the grapes for each solution (water, grape juice, corn syrup)? 2. Is there evidence of osmosis in the grapes? Explain briefly. 3. Based on your observations of the grapes in the solutions, which solutions were a) hypertonic = b) hypotonic = c) isotonic = and how do you know? Explain. Was this what you expected? Explain. 4. Use a (rough) bar graph to illustrate the data/results of your groups’ data [for each solution (water W, grape juice G, corn syrup CS) compare: initial mass (I.) and final mass (F.)]. I. F. W I. F. G I. F. CS 5. How does the data of the other groups compare to yours? (similar, different, be specific). 6. Discuss and state what could be done differently in the future to alter the results or experiment in any way (one possible example). Grape weight (g) after being in 50 ml of: Group A water grape juice corn syrup period 1 4.79 5.33 4.74 period 2 4.87 5.35 4.85 period 7 4.95 5.36 4.7 day 6 5.35 5.37 4.46 Grape weight (g) after being in 100 ml of: Group B water grape juice corn syrup period 1 4.05 5.13 5.86 period 2 4.07 5.15 5.81 period 7 4.15 5.16 5.78 day 6 4.45 5.27 5.36 Grape weight (g) after being in 150 ml of: Group C water grape juice corn syrup period 1 4.54 4.58 4.71 period 2 5.47 5.5 5.53 period 7 4.74 4.74 4.65 day 6 5.06 5.72 4.32