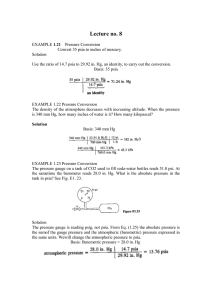
Metric Sample Problems
advertisement
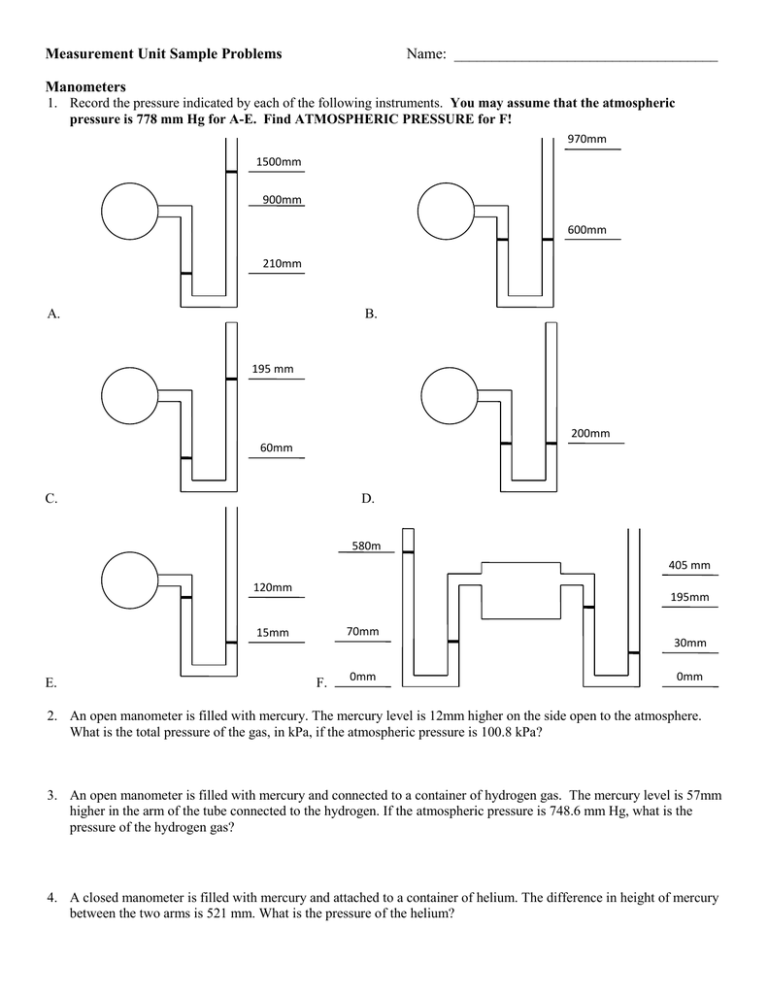
Measurement Unit Sample Problems Name: ___________________________________ Manometers 1. Record the pressure indicated by each of the following instruments. You may assume that the atmospheric pressure is 778 mm Hg for A-E. Find ATMOSPHERIC PRESSURE for F! 970mm 1500mm 900mm 600mm 210mm A. B. 195 mm 200mm 60mm C. D. 580m m 120mm 195mm 70mm 15mm E. 405 mm F. 0mm 30mm 0mm 2. An open manometer is filled with mercury. The mercury level is 12mm higher on the side open to the atmosphere. What is the total pressure of the gas, in kPa, if the atmospheric pressure is 100.8 kPa? 3. An open manometer is filled with mercury and connected to a container of hydrogen gas. The mercury level is 57mm higher in the arm of the tube connected to the hydrogen. If the atmospheric pressure is 748.6 mm Hg, what is the pressure of the hydrogen gas? 4. A closed manometer is filled with mercury and attached to a container of helium. The difference in height of mercury between the two arms is 521 mm. What is the pressure of the helium? Dimensional Analysis 5. 450 000 mm = ? hm 6. 2.3 x 10-8 kL = ? µL 7. 3.6 x 104 s = ? days 8. 36 mm3 = ? cm3 9. A room measures 144 in by 180. in. Calculate the number of square yards of carpet needed to cover this area. 10. A motorcycle is clocked at 30.6 m/s. Will the driver get a ticket if the speed limit is 55 mph? Density 11. What is the density (in kg/L) of a rock if its mass is 36 g and its volume is 12 cm3? 12. If a block of wood has a density of 0.60 g/mL and a mass of 120. g, what is its volume? 13. A sample of aluminum has a volume of 45.0 mL. Calculate the mass if aluminum has a density of 2.70 g/mL. Specific Heat 14. It takes 487.5 J to heat 25 grams of copper from 25 °C to 75 °C. What is the specific heat of copper? 15. How much energy is required to heat 120.0 g of water from 2.0 °C to 24.0 °C? 16. Sidney is home from school with a cold, so mom has made him a bowl of chicken soup (c=4187J/kg°C) which she ladles from a pot into a glass bowl. If 0.60 kg of soup at 90.°C is placed in a 0.20 kg glass bowl (c=840J/kg°C) that is initially at 20.°C, what will be the temperature of the soup when the soup and the bowl have reached equilibrium?