Chemistry Measurement Worksheet: Scientific Notation & More
advertisement

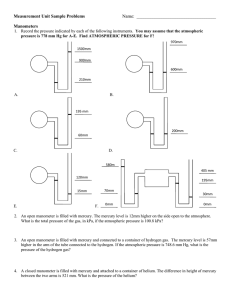
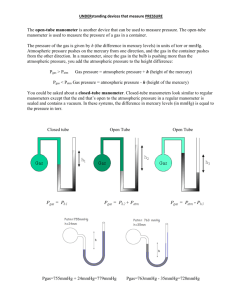
Measurement Chemistry 6.0 Name________________________________ Scientific Notation 1. Write each of the following in common (expanded) form: a. 2.63 x 101 ______________________ b. 7.01 x 100 ______________________ c. 4.33 x 106 ______________________ d. 2.21 x 10-5 ______________________ 2. Write each of the following in scientific (exponential) notation: a. 8020000 ______________________ b. 0.0264 ______________________ c. 4.23 ______________________ d. 0.00020 ______________________ 3. Calculate each of the following and express the answer in correct scientific notation: a. 7.1 x 102 + 4.5 x 103 ______________________ b. 4.6 x 10-5 - 7.3 x 10-4 ______________________ c. (3.0 x 102)(5.0 x 10-3) ______________________ d. (4.1 x 10-3)(3.0 x 10-2) ______________________ e. (1.2 x 10-4) /( 3.0 x 10-1) ______________________ f. (6.4 x 103) / (8.0 x 101) ______________________ g. 1.44 x 108 ______________________ 2 Uncertainty in Measurements 4. Read the level of the liquid in each of these graduated cylinders to the nearest 0.01 mL. Remember to read the volumes at the meniscus. __________ ___________ 5. An electronic balance shows the mass of a sample of sodium chloride to be 29.732 g. What is the uncertainty of the measurement? _________________ 6. Record the mass represented below. _________________ 3 7. Record the measurement value for each graduated cylinder to the proper precision. Include units. 4 8. Record the Celsius temperature for each thermometer. 5 Significant Figures 9. How many significant figures are in the following measurements a. 8.5 m ________ b. 0.0007 g ________ c. 190 kg ________ d. 5020 cL ________ e. 1.0003 dm ________ f. 0.00003020 hm________ g. 3004000 mg ________ h. 6.30 x 109 Gs ________ i. 1.00 s j. 300. daL ________ ________ 10. Express each of the following measurements with 3 significant figures. a. 1.986 m _____________ b. 0.07787 L ______________ c. 1543 ms _____________ d. 19000000 ng ______________ 11. Add or Subtract: Express answers with the correct number of significant figures. Include units in the answer. a. 111 m + c. 120 g + 0.8 m 0.96 g b. d. 0.6598 nm - 0.321 nm 13.793 L - 12.8 L 12. Multiply or Divide: Express answers with the correct number of significant figures. a. 3.2 cm x c. 1.09 mL x 4.92 cm 13.5 g/mL b. 0.0872 m3 d. 25.00 g ÷ 2.1960 m2 ÷ 6 g/mL 6 Manometers A manometer is a laboratory device used to measure the pressure of a gas. The gas in the bulb expands and exerts a pressure on the mercury inside of the u-tube. The greater the pressure, the higher up the tube the mercury is forced. A closed manometer is one where the tube is sealed off so the atmospheric pressure is not a factor in the final positions of the mercury levels. The height (h) is the difference between the mercury levels and represents the pressure of the gas. An open manometer is one where the tube is open to the atmosphere. The gas pressure is attempting to force the mercury up the tube while the atmospheric pressure is attempting to force the mercury down the tube. The height (h) represents the difference in pressures between the gas and the atmosphere. Note: 1 atm = 760 mm Hg 13. If the difference in mercury levels (h) in each of the three manometers pictured above is 75.0 mm, determine the pressure of the gas in each. (a) (b) (c) 775mm 465mm 50mm 420mm 0mm 0mm 7 14. A gas container is fitted with a manometer. The level of mercury is 15.33 mm lower on the open side. Using a laboratory barometer, you find that atmospheric pressure is 750.0 mmHg. What is the pressure of the gas in the container? 15. A soccer ball is attached to an open-ended manometer. The mercury level in the manometer is 10.1 mm higher on the side attached to the ball than on the side open to the atmosphere. Atmospheric pressure has already been determined to be 770. mmHg. What is the gas pressure of the ball? 16. A gas container is fitted with a closed-ended manometer. The level of mercury is 21.5 mm lower on the side closest to the gas. If the barometric pressure is 747.39 mmHg, what is the pressure of the gas in the container? 17. One end of an open-ended manometer is connected to a canister filled with a gas at a pressure of 771 mmHg. The mercury level on the side open to the atmosphere is 11.29 mm higher than on the side connected to the canister. What is the atmospheric pressure? Temperature Conversions 18. Convert Celsius temperatures to Kelvin and Kelvin temperatures to Celsius. a. 13.21°C b. 743.0 K c. 115 K d. −39.9°C e. 1209.84 K f. 208°C 8 Metric System 19. Provide the metric system unit and prefix that would be the best to use when measuring the following: a. The length of your finger: ____________ b. The mass of a peanut: ____________ c. The volume of a glass of soda: ____________ d. The length of your basement: ____________ e. The height of Mount Everest: ____________ f. The mass of a horse: ____________ g. The volume of water in a bath tub: ____________ 20. Of the following measurements, circle the one that is the largest. a. 5 km or 5 mm b. 12 dg or 12 dag c. 1 m or 50 cm 21. Of the following measurements, circle the one that is the smallest. a. 10 m or 10 hm b. 25 cg or 25 dag c. 2 km or 5000 mm Dimensional Analysis The following conversions must be done using dimensional analysis. The process is considerably more important than the answer. Work must be shown with units clearly labeled throughout the problem. Be sure to include the proper number of significant figures in your answer. 22. Convert the following metric measurements to the indicated unit. a. 5 ms = b. 25 g = _________________ s _________________ kg 9 c. 0.005 hL = _________________ L d. 6.4 g = _________________ hg e. 0.000941 kL = _________________ L f. 13 dag = _________________ dg g. 0.72 cm = _________________ mm 23. The air pressure for a certain tire is 109 kPa. What is the pressure in atmospheres? 24. Convert 16.9 inches to feet. 10 25. Convert 195 J to calories. 26. Convert 18.7 mm2 to dm2 27. The air pressure inside a submarine is 0.62 atm. What would be the height of a column of mercury balanced by this pressure? 28. How many liters are in 2.9 pts? 29. Convert 2.58 s to ns. 30. Convert 120 cal to Joules. 31. The weather news gives the atmospheric pressure as 1.07 atm. What is this atmospheric pressure in mm Hg? 32. How many ounces are in 327 dag? 33. Convert 3.1 miles to inches. 11 34. Convert 31.5 m/s to mi/hr 35. An experiment at Sandia National Laboratories in New Mexico is performed at an atmospheric pressure of 758.7 mmHg. What is this pressure in atm? 36. Convert 11.9 ounces to cg. 37. Convert 3.2 x 105 cal to kilojoules. 38. Convert 3.98 ng to decagrams. 39. A bag of potato chips is sealed in a factory near sea level. The atmospheric pressure at the factory is 761.3 mm Hg. The pressure inside the bag is the same. What is the pressure inside the bag of potato chips in Pa? 40. Convert 8.62 x 10-6 GL to m3 41. Convert 1.39 lbs to g. 12 2 42. The pressure tank on a compressed air tank reads 43.2 lb/in . What is the pressure in atm? 43. How many milliliters of milk are in a standard school milk carton (0.50 pints)? 44. Convert 595 km to megameters. 45. Convert 13.6 g/cm3 to lbs/in3 46. How many ounces are in 14.88 Gg? 47. Convert 395 kJ to kcal. 48. Convert 187 ft to km. 49. Determine the volume of a block in cm3 with dimensions 3.1 in x 2.8 in x 4.67 in. 13 −8 50. Convert 2.12 x 10 cm/min to fathoms per fortnight given the following conversion factors. 1 in. = 2.54 cm 1 fortnight = 14 days 1 fathom = 6 ft 51. If 1.0 x108 sodium atoms could be arranged side by side, they would form a straight line measuring 3.1cm. What is the diameter of 1 sodium atom in Angstroms? (1 Å = 10-10 m) Density 52. Determine the density of the following objects. Indicate if they will sink or float in water. a. m = 11.9 g V = 37 mL b. An object weighing 0.26 g and occupying a volume of 0.89 cm3. c. A 46 mL stone that weighs 250 g. 53. Determine the mass of a 13.6 mL object with a density of 2.65 g/mL. 54. What is the volume of 942.5 g of iron? The density of iron is 7.86 g/mL. 14 3 3 55. Gold has a density of 19.3 g/cm . What is the mass of 3.4 m of gold? 56. Calculate the mass, in pounds, of 29.8 mL of aluminum, which has a density of 2.70 g/mL. 57. Determine the volume, in pints, of 0.498 kg of a liquid that has a density of 0.754 g/mL. Specific Heat Capacity 58. How much heat is required to raise the temperature of 3.90 g of liquid water by 45°C? 59. Calculate the specific heat of a substance if the temperature of a 28.4 g sample increases from 10.0°C to 28.1°C when 207 J of heat is added. 60. A 15.9 g sample of aluminum has a temperature of 18.7°C. What will the temperature of the aluminum be when 84.3 cal of heat are added? 15 61. When 15.4 g of solid steel is heated at 49.3°C by adding 40.5 J energy, what is the final temperature of the metal? 62. How many calories of heat are required to raise the temperature of 157 g of hexane from 32.4°C to 34.7°C? 63. Explain why deserts experience extreme temperatures while islands experience moderate temperatures. Review 64. Write the following in scientific notation: a. 0.0000652 b. 120000000 65. Write the following in expanded form (long form) a. 3.6 x1015 b. 5.99 x10−5 66. How many significant figures are in the following numbers: a. 100 b. 0.000200 c. 189 e. 0.0000001 f. 1.00000010 c. -23 6700. x 10 c. 530. x 10−5 d. 4.60 x10−8 g. 60003000 h. 1200. 67. Round the following numbers to three significant figures. a. 1253 b. 0.89562 c. 20 d. 10000 16 2 68. The pressure in the tire of an automobile is 34.8 lb/in ? What is the pressure in kPa? 69. Suppose you are measuring the pressure inside a sealed cabinet using the open-ended manometer. The atmospheric pressure is 762.4 mm Hg. If the mercury level on the side open to the atmosphere is 3.6 mm higher than on the side attached to the cabinet, what is the pressure inside the cabinet in units of kPa? 70. A water sample has a temperature of 25.3°C. What is the temperature in Kelvin? 71. The temperature of a gas is determined to be 97.65 K. What is this temperature in degrees Celsius? 72. A manometer contains a sample of nitrogen gas at a pressure of 88.3 kPa. The level of mercury in the U-tube is 12.8 mm lower on the end open to the atmosphere. What is the atmospheric pressure in kPa? 73. How many millimeters are in 4.69 miles? 74. Convert 24.09 pts to liters 75. How many ounces are in 2.5 g of water? 17 6 3 76. The volume of a wood block is determined to be 1.649 × 10 mm . What is the volume of the block in ft3? 77. Perform the following conversions: a. 3.9 dg = _____________cg b. 14 km = ______________Gm c. 2.5 x1012 nL = ___________L d. 47.11 s = _____________Ms 78. Explain why all measurements are considered to be uncertain. How is this related to significant figures? 79. Determine the density of an object with a mass of 1.67 g and a volume of 13.8 mL. Will it sink or float in water? 80. A metal rod is found to have a density of 7.19 g/mL with a mass of 26.3 g. What is its volume? 81. Mercury has a density of 13.6 g/mL. What is the mass of 67.44 mL of mercury? 18 82. Tin has a density of 7.31 g/cm . A piece of tin weighing 0.357 oz is placed in a graduated cylinder containing 21.3 mL of water. If the tin is completely submerged, what is the final reading on the graduated cylinder for the water volume? 3 83. A student calculates the density of iron as 6.94 g/cm3 using lab data for mass and volume. A handbook shows that the correct value is 7.86 g/cm3. Calculate the percent error. 84. A block of sodium with measurements 3.00 cm x 5.00 cm x 5.00 cm has a measured mass of 75.5 g. The accepted density for sodium if 0.971 g/cm3. Calculate the percent error. 85. Aluminum foil on a certain roll has a total area of 18.5 m2 and a mass of 1275 g. Using the density of 2.70 g/cm3 for aluminum, determine the thickness, in millimeters, of the aluminum foil. 86. If 159 J of heat are added to an unknown metal of mass 135 g, the temperature of the metal goes from 25.2°C to 27.3°C. Calculate the specific heat of the metal. 87. A 47.6 gram sample of tin at 85.0°C is placed into 150. grams of water. The water and tin reach thermal equilibrium at a temperature of 32.1°C. What was the initial temperature of the water?