O Level Physics Chap 19 Density and Pressure
advertisement

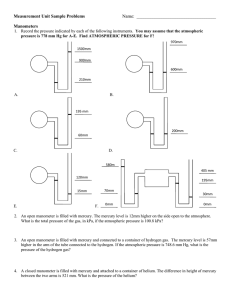
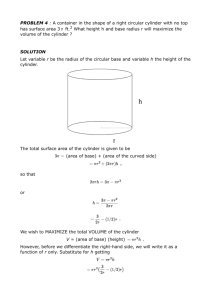
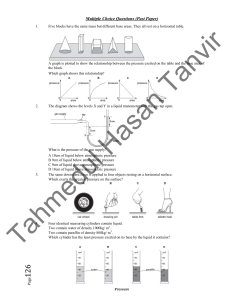
Prepared By: Shakil Raiman Density: Density of a substance is defined as its mass per unit volume. Unit: kg/m3, g/cm3 For Regular Solid: Measure the mass of the object. Measure the dimensions. (Suppose for a cuboid length, width, and height) Calculate volume by using dimensions. (Suppose for a cuboid Volume = length×width×height) Calculate density by using formula density = mass/volume For Irregular Solid: Measure the mass of the object. Take some suitable liquid within a measuring cylinder. The liquid should be enough so that the object sink completely. Measure the volume of the liquid. (V1) Put the object into the liquid of the measuring cylinder. Measure the new volume of the liquid level. (V2) Volume = V2 – V1 Calculate density. (density = m/ (V2 – V1)) For Irregular Solid: Measure the mass of the object. Take some suitable liquid within a displacement can. Wait for no liquid fall for the cal. Put the object into the liquid and use measuring cylinder to measure the displaced liquid Calculate density by using formula, density = mass/ volume. For Liquid: Take a measuring cylinder. Measure the mass of it. (m1) Take the liquid within measuring cylinder. Read the volume of the liquid level. (V) Measure the mass of the measuring cylinder with the liquid. (m2) Mass of liquid = m2 – m1 Calculate density by using formula, density = mass/volume Mass: Mass of a body is the measure of the amount of matter that it contains. Weight: Weight of a body is the attractive force exerted on it due to gravity. Inertia: Any object tends to remain in its own state of rest or motion. Such tendency is called inertia. Mass of an object is always the same Weight of an object varies from place to place is a measure of inertia is measured using a beam balance or an electronic balance. is a measure of force is measured using a spring balance or a compression balance is measured in kg is measured in N mass: 1 kg Weight on Earth: 10 N Weight on Moon: 1.6 N Pressure is force acting normally per unit area. Pressure = Force / Area Unit: Pascal (Pa), Nm-2, Ncm-2 If area is constant, Pressure increases if force increases and pressure decreases if force decreases. If force is constant, pressure increases if area decreases pressure decreases if area increases We have air around us and therefore there is atmospheric pressure. Atmospheric pressure exists because of molecular bombardment of energetic molecules. The normal atmospheric pressure is about 1.013×105 Pa. It is called 1 atmosphere ( 1 atm. ) The pressure due to a liquid column depends on the depth, height and the density of liquid. Liquid Pressure = height × density × g P=hg Mass of water = volume × density = 𝐴ℎ Force = Weight of Liquid Pressure = 𝐴ℎ𝑔 So, 𝑃𝑟𝑒𝑠𝑠𝑢𝑟𝑒 = 𝐹𝑜𝑟𝑐𝑒 𝐴ℎ𝜌𝑔 = 𝐴𝑟𝑒𝑎 𝐴 =ℎ𝜌𝑔 Open end: Pressure at X = P0+hpg Close end: Pressure at Y = hpg The pressure in a liquid depends on 1. the density of the liquid 2. the depth of the liquid Note: the pressure in liquid does not depend on the area at any depth. In the following picture, there is equal pressure at point A, B or C. Simple mercury barometer is used to measure atmospheric pressure. Construction 1. a thick-walled glass tube ( about 1m long) is filled with mercury completely 2. the open end of the is covered with a finger and inverted 3. place the inverted tube in a mercury container Observation: The height of the mercury column is found to be about Atmospheric pressure=760 mmHg or 76 cmHg Manometer is used to measure gas pressure. Construction: The manometer consists of a U-tube containing a column of liquid. The liquid can be mercury, water or oil. Pressure of Gas = P0+hpg Pressure can be transmitted through a liquid in hydraulic presses and hydraulic brakes on vehicles. A small force on smaller piston can cause greater force on greater piston. Wish you all very good luck and excellent result.