Lecture no. 8
advertisement
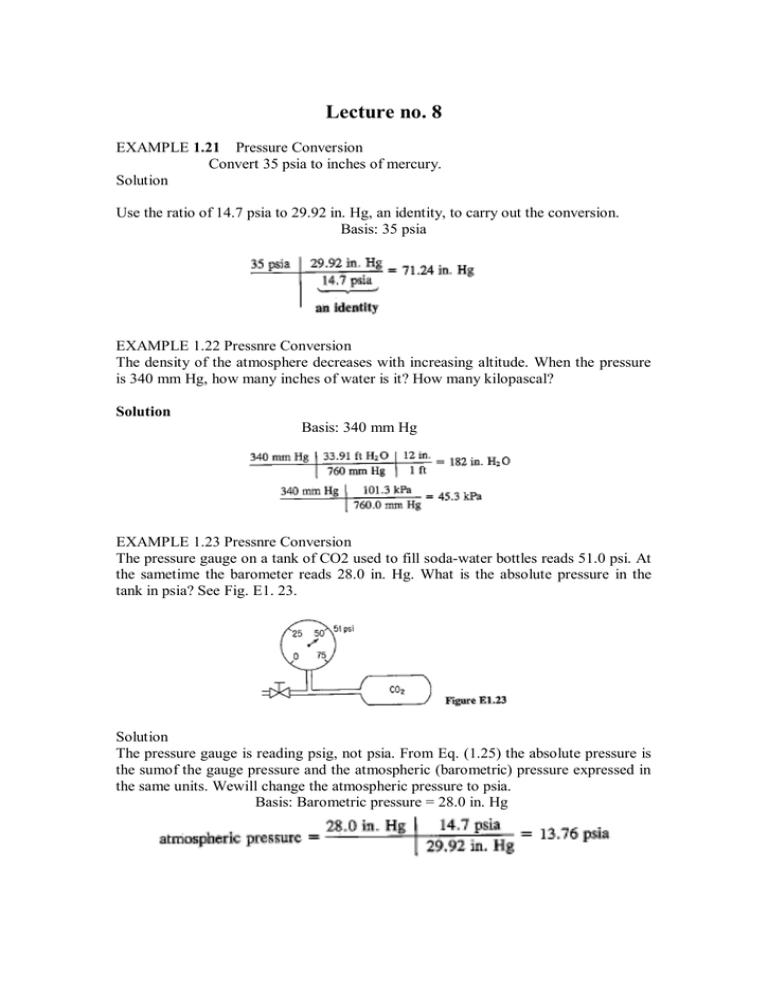
Lecture no. 8 EXAMPLE 1.21 Pressure Conversion Convert 35 psia to inches of mercury. Solution Use the ratio of 14.7 psia to 29.92 in. Hg, an identity, to carry out the conversion. Basis: 35 psia EXAMPLE 1.22 Pressnre Conversion The density of the atmosphere decreases with increasing altitude. When the pressure is 340 mm Hg, how many inches of water is it? How many kilopascal? Solution Basis: 340 mm Hg EXAMPLE 1.23 Pressnre Conversion The pressure gauge on a tank of CO2 used to fill soda-water bottles reads 51.0 psi. At the sametime the barometer reads 28.0 in. Hg. What is the absolute pressure in the tank in psia? See Fig. E1. 23. Solution The pressure gauge is reading psig, not psia. From Eq. (1.25) the absolute pressure is the sumof the gauge pressure and the atmospheric (barometric) pressure expressed in the same units. Wewill change the atmospheric pressure to psia. Basis: Barometric pressure = 28.0 in. Hg (Note: Atmospheric pressure does not equal 1 standard atm.) The absolute pressure in the tank is 51.0 + 13.76 = 64.8 psia In some instances the fluids in the legs of the manometer are not the same. Examine Fig. l.l3. When the columns of fluids are at equilibrium (it may take some timel) the relation between PI, p, and the heights of the various columns of fluid is Can you show for the case in which PI = p, = p that the manometer expression reduces to Finally, suppose that fluids I and 3 are gases. Can you ignore the gas density p relative to the manometer fluid density? For what types of fluids? EXAMPLE 1.24 Pressure Conversion Air is flowing through a duct under a draft of 4.0 em HzO. The barometerindicates that the atmospheric pressure is 730 mm Hg. What is the absolute pressure of the gas in inches of mercury? See Fig. E1.24. Solution Wecan ignore the gas density abovethe manometer fluid. In the calculations we have to employ consistent units, andit appears in this case thatthe most convenient units are those of inches of mercury. Basis: 730 mm Hg Basis: 4.0 em H,O draft (under atmospheric) Whatis another way to makethe conversion? Since the reading is 4.0 em H,O draft (under atmospheric), the absolute reading in uniform units is 28.7 - 0.12 = 28.6 in. Hg absolute EXAMPLE 1.25 Vacuum Pressure Reading Small animals such as mice can live at reduced air pressures down to 20 kPa (although not comfortably). In a test a mercury manometer attached to a tank as shown in Fig. E1.25 reads 64.5 cm Hg and the barometer reads 100 kPa. Will the mice survive? Basis: 64.5 em Hg below atmospheric We ignore any temperature corrections to convert the mercury density and also ignore the gas density above the manometer fluid. Then, since the vacuum reading on the tank is 64.5 cm Hg below atmospheric, the absolute pressure in the tank is The mice probably will uot survive. EXAMPLE 1.26 Calculation of Pressure Difference In measuring the flow of fluids in a pipeline. a differential manometer, as shown in Fig. E1.26, can be used to determine the pressure difference across an orifice plate. The flow rate can be calibrated with the observed pressure drop. Calculate the pressure drop P 1 – P 2 in pascal. Solution In this problem we cannot ignore the water density above the manometer fluid. Apply Eq.(1.26b), as the densities of the fluids above the manometer fluid are the same. P 1 – P 2 = (p f - p)gd