File
advertisement

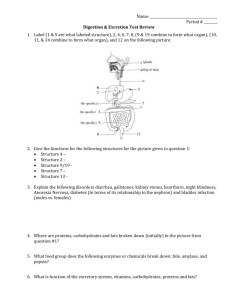
Term 1 Molecules in motion After Intro to Biology Scientific method, taking notes, Harvard Referencing, Using the Microscope Macromolecules: Page 13 Heinemann Inorganic Molecules-water, carbon dioxide, nitrogen and minerals Organic Molecules-complex compounds that contain carbon and hydrogen STUDENTS- read and make own notes on the composition of organisms plus water and oxygen/carbon dioxide, nitrogen and minerals Year 11 Chemistry of Life What chemicals do organisms need or make? Water (65-70% of the body) Proteins Fats Carbohydrates (These are all organic Substances) Carbohydrates Carbohydrates are the most abundant biological molecules, and fill numerous roles in living things such as; Storage and transport of energy (Starch and glycogen) and structural components (cellulose in plants in chitin animals) They contain elements carbon, hydrogen and oxygen The basic carbohydrate units are called monosaccharides, such as glucose, galactose and fructose. Two joined monosaccharides are called disaccharides, such as sucrose and lactose. Anything larger than this is a polysaccharide. Polysaccharides, such as starch, glycogen or cellulose, can reach many thousands of units in length. Note: ordinary carbohydrates are made of a glucose molecule linked to another monosaccharide like fructose-to form a disaccharide. Fats Fats are made of carbon, hydrogen and oxygen too, but they contain more carbon and hydrogen than they do oxygen- so it is the proportions of the elements which change the substance. Fats may either solid or liquid at normal room temperature, depending on their structure and composition. They consist of a wide group compounds that are generally soluble in organic solvents and largely insoluble in water. Main part glycerol and attached to this are chains called fatty acids, which can be split to have the separate parts by adding water (hydrolysis) If we remove water they link-condensation Fats give us warmth/insulation Energy stores Structure of a carbohydrate and fats Proteins Proteins also contain carbon, hydrogen, and oxygen and nitrogen- sometimes they contain sulphur. Like other biological macromolecules such as polysaccharides; proteins are essential parts of all living organisms and participate in every process within cells. Proteins are relatively large organic compounds made of amino acids arranged in a linear chain and joined together by peptide bonds There are about 20 amino acids in nature The particular amino acids present and the order in which they occur vary from one protein to another. In a protein the chain of amino acids is coiled and folded to form a protein The protein can be broken back down by breaking the bonds first by hydrolysis- to get the polypeptides, then by condensation- amino acids individually Proteins cont: Proteins are found in various structures Hair Bones Muscles Blood Soluble proteins-enzymes Can’t heat a protein above 40 degrees or denatures and can’t function Chemical reactions in the body occur in two places the gut and the cells! Reactions in the cells are called metabolism Some reactions build things up – Glucose-to multi sugars-glycogen to be stored Or broken down-glucose for energy, water, carbon dioxide Reactions are catalysed by enzymes Warmer the temperature the faster processes go until over the 40 degrees- denaturing Within cells reactions take place in the watery solutionthat’s why we need lots of water! Students Need to write own notes on nucleic acids & vitamins HOMEWORK: KEY QUESTIONS PAGE 17 AND CHAPTER REVIEW ***YOU NEED TO RESEARCH A DISEASE THAT RESULTS AS A LACK OF EITHER A VITAMIN (b12) OR A MINERAL (Calcium) and produce at least a half page word doc and be prepared to read out to the class next lesson.