Quiz 3 – BONUS
advertisement

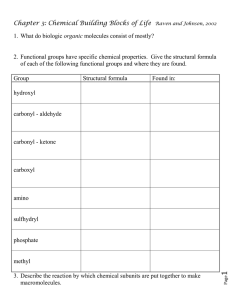
Quiz 3 – BONUS 5 points Due Monday, Feb 1, 2016 at 9:30am sharp This quiz is to be turned in at the beginning of class – NO EXCEPTIONS. That means, before I begin lecturing. If you arrive late to class, I will not accept your quiz, so you might as well not bother turning it in. This is not intended to give away free points. This is meant to ensure that you read chapter 3 and show me that you have an understanding of the material. All answers can easily be found in Chapter 3. An easy way to find answers is to look at headings, bolded terms, and tables. I do not have specific points assigned to each question. Simply, if you answer all the questions correctly, you will receive full credit. If you answer half the questions you will receive half credit, etc. Ready? Begin! 1. Why are C—C and C—H molecules considered nonpolar? 2. Define the following terms: a) functional group (give an example): b) isomer: c) polymer: d) monomer: e) hydrolysis: f) dehydration: g) peptide bond: h) polypeptide: 3. Give the structure of the following structural groups and list where each can be found: a) Hydroxyl: b) Carbonyl: c) Carboxyl: d) Amino: e) Sulfhydryl: f) Phosphate: g) Methyl: 4. List the four major biological macromolecules: 1. 2. 3. 4. 5. Give one function for each of the following macromolecules AND an example for each: a) starch: function _________________________ example ____________________ b) chitin: function _________________________ example ____________________ c) DNA: function _________________________ example ____________________ d) RNA: function _________________________ example ____________________ e) functional proteins: function _________________________ example ____________________ f) structural proteins: function _________________________ example ____________________ h) fats: function _________________________ example ____________________ i) steroids: function _________________________ example ____________________ 6. For the following molecules, state whether they are mono-, di-, or polysaccharides: a) Glucose: _________________________ b) Fructose: _________________________ c) Sucrose: __________________________ d) Amylose: _________________________ 7. List two other polysaccharides (hint: one is used by plants and the other by insects and fungi for structural support): a) b) 8. What are the two main varieties of nucleic acids (do not use the acronyms)? a) b) 9. What are the main differences between RNA and DNA? 10. List two roles of RNA: a) b) 11. Which nucleotide-containing molecule is responsible for energy in living systems? _____________________________ 12. List seven functions of proteins: a) b) c) d) e) f) g) 13. Draw the general structure of an amino acid. Indicate which are the carboxyl, amino, and R groups. 14. The covalent bond that links two amino acids is called a __________________ ___________________. 15. Name the four levels of structure of proteins: a) b) c) d) 16. What are the two kinds of secondary structures called? 17. What are two additional structural characteristics? a) b) 18. What type of special proteins are necessary for folding of proteins? 19. What is denaturation? 20. Thinking about fats: a) What do they consist of? b) What are the three types of fatty acids? c) The main function of fats is ________________________. 21. Complex lipid molecules called ___________________________________ are among the most important molecules of the cell because they form the core of all biological membranes.