Solubility and Precipitation Rules
advertisement

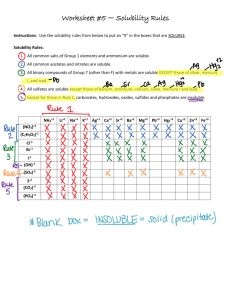
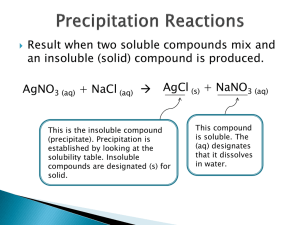
The ability to dissolve or break down into its component ions in a liquid Example: NaCl is soluble Completely dissolves in water AgCl is insoluble Stays a solid in water Li, Na, K, NH4, NO3 are all soluble Therefore, anything that contains these ions are soluble Cl, Br, and I are soluble except when paired with Ag, Hg2, and Pb SO4 is soluble except with Sr, Ba, Pb, or Ca OH, S, CO3, PO4 are insoluble except when paired with Li, Na, K, NH4 Determine whether each of the follow compounds is soluble or insoluble: AgBr Insoluble – Br is normally soluble but not when its with Ag CaCl2 Soluble Pb(NO3)2 Soluble – NO3 is always soluble NH4Cl Soluble - NH4 and Cl are both soluble A reaction in which a solid is formed upon mixing two aqueous solutions Example: Potassium Iodide + Lead Nitrate 2 KI (aq) + Pb(NO3)2 (aq) -> 2 KNO3 (aq) + PbI2 (s) Write an equation for the precipitation reaction that occurs (if any) when solutions of sodium carbonate and copper (II) chloride are mixed. Na2CO3 (aq) + CuCl2 (aq) -> Write an equation for the precipitation reaction that occurs (if any) when solutions of lithium nitrate and sodium sulfate are mixed. Li2NO3 (aq) + Na2SO4 (aq) -> Molecular Formula An equation that shows the complete neutral formula for every compound AgNO3 (aq) + NaCl (aq) -> AgCl (s) + NaNO3 (aq) Complete Ionic Equation An equation that shows the reactants and products as they are present in solution Ag+ (aq) + NO3- (aq) + Na+ (aq) + Cl- (aq) -> AgCl (s) + Na+ (aq) + NO3- (aq) Spectator Ions Ions that are present on both the reactant and product side. They do not participate in the reaction Ag+ (aq) + NO3- (aq) + Na+ (aq) + Cl- (aq) -> AgCl (s) + Na+ (aq) + NO3- (aq) Net Ionic Equation An equation that eliminates the spectator ions and shows only the elements or compounds that are participating Ag+ (aq) + Cl- (aq) -> AgCl (s) Consider the following reaction: HCl (aq) + NaOH (aq) -> H2O (l) + NaCl (aq) What is the complete ionic equation? Which ions are the spectators? What is the net ionic equation?