Mg(s) + 2 H + (aq)
advertisement

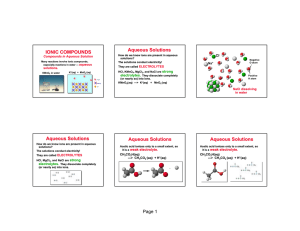
CCR, page 177 An Ionic Compound, CuCl2, in Water Aqueous Solutions How do we know ions are present in aqueous solutions? The solutions conduct electricity! They are called ELECTROLYTES HCl, CuCl2, and NaCl are strong electrolytes. They dissociate completely (or nearly so) into ions. Aqueous Solutions Acetic acid is a weak acid, it ionizes only to a small extent, so it is a weak electrolyte. CH3CO2H(aq) <---> CH3CO2-(aq) + H+(aq) ACIDS Acids ---> H+ in water HCl(aq) HCl is a strong acid ---> H+(aq) + Cl-(aq) Other strong acids include: HI, HBr, HNO3, H2SO4, HClO4, HClO3 Weak acids have H+ as the cation, and are not STRONG acids. BASES Base ---> OH- in water NaOH(aq) NaOH is a strong base ---> Na+(aq) + OH-(aq) Other strong bases include: LiOH, KOH, RbOH, CsOH, Ca(OH)2, Ba(OH)2, Sr(OH)2 Weak bases have OH- as the anion, and are not STRONG bases. Ammonia, NH3 An Important Weak Base Memorize Ammonia, NH3, as a Weak Base! Aqueous Solutions Some compounds dissolve in water but do not conduct electricity. These are molecular compounds, made of molecules, so they do not split into charged particles. They are called nonelectrolytes. Examples include: sugar ethanol ethylene glycol KISS Rules – Keep it Simple Solubility Rules #1 – Soluble Cations: sodium, potassium, and ammonium are always soluble #2 – Soluble Anions – nitrate, acetate, chlorate, perchlorate are always soluble #3 – Soluble Anions – chloride, bromide, iodide are soluble EXCEPT with the SILVER GROUP – silver, mercury and lead #4 – Soluble Anions – sulfate is soluble EXCEPT with the Silver Group or with strontium or barium #5 – EVERYTHING ELSE IS INSOLUBLE Net Ionic Equations Mg(s) + 2 HCl(aq) --> H2(g) + MgCl2(aq) Use your knowledge of Strong Electrolytes to determine what is Aqueous: HCl is a strong acid, so it completely dissociates in water and can be written as separate ions to show how it actually exists. MgCl2 is soluble (KISS rules), so it completely dissociates in water and can be written as separate ions to show how it actually exists. We really should write: Mg(s) + 2 H+(aq) + 2 Cl-(aq) ---> H2(g) + Mg2+(aq) + 2 Cl-(aq) The two Cl- ions are SPECTATOR IONS — they do not participate. *Could have used NO3- instead and the reaction would not have changed! Net Ionic Equations Mg(s) + 2 HCl(aq) --> H2(g) + MgCl2(aq) Mg(s) + 2 H+(aq) + 2 Cl-(aq) H2(g) + Mg2+(aq) + 2 Cl-(aq) We leave the spectator ions out — Mg(s) + 2 H+(aq) ---> H2(g) + Mg2+(aq) to give the NET IONIC EQUATION Precipitation Reactions The “driving force” is the formation of an insoluble compound — a precipitate. Pb(NO3)2(aq) + 2 KI(aq) 2 KNO3(aq) + PbI2(s) Net ionic equation Pb2+(aq) + 2 I-(aq) PbI2(s)