Precipitation Reactions: Chemistry Presentation
advertisement
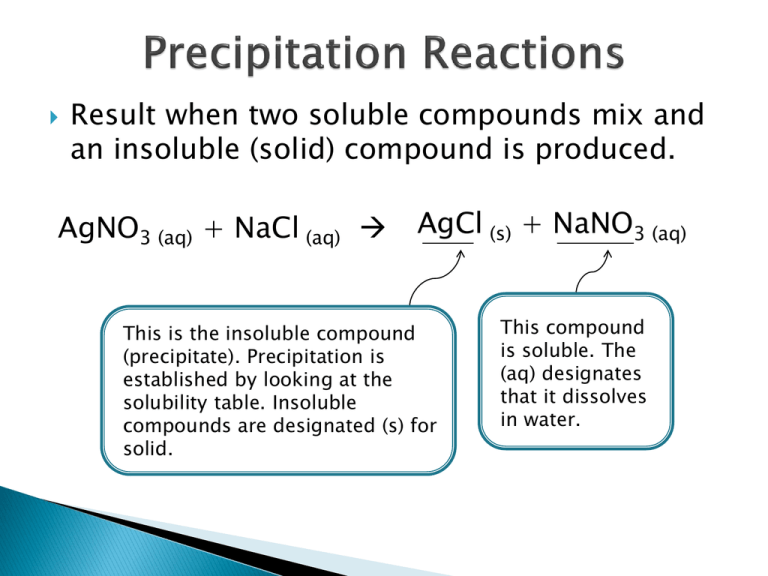
Result when two soluble compounds mix and an insoluble (solid) compound is produced. AgNO3 (aq) + NaCl (aq) AgCl (s) + NaNO3 (aq) This is the insoluble compound (precipitate). Precipitation is established by looking at the solubility table. Insoluble compounds are designated (s) for solid. This compound is soluble. The (aq) designates that it dissolves in water. We predict the products by matching the cation of one reactant to the anion in the other reactant, and vice versa. silver cation + chloride anion AgNO3 (aq) + NaCl (aq) nitrate anion + sodium cation AgCl (s) + NaNO3 (aq) Step 1. Check to see if a precipitate forms using the solubility table. this is insoluble Ca(NO3)2 (aq) + NaF (aq) this is soluble Step 2. Write the products with cations and anions in a 1:1 ratio. this is insoluble Ca(NO3)2 (aq) + NaF (aq) CaF (s) + NaNO3 (aq) this is soluble One calcium ion to one fluoride ion. The (s) is added because this is the insoluble compound. One sodium to one nitrate ion. Nitrate is a polyatomic ion; we do not alter it’s formula. The (aq) is used for the soluble compounds. Step 3. Determine the charges of each ion on the product side, and balance the products using subscripts. +2 -1 2 +1 -1 Ca(NO3)2 (aq) + NaF (aq) CaF2 (s) + NaNO3 (aq) Since these charges did not balance out, a subscript of 2 was added to fluoride so the total charges would add to zero. Since these charges do balance out, no subscript is needed. Step 4. Balance the equation using coefficients. +2 -1 2 +1 -1 Ca(NO3)2 (aq) + 2 NaF (aq) CaF2 (s) + 2 NaNO3 (aq) These are added to balance the equation. The total ionic equations represents what each compound looks like when dissolved in water. Strong electrolytes break apart 100% in water to form ions, so these are shown as ions in the equation. ◦ NaCl (aq) Na+ (aq) + Cl- (aq) Weak electrolytes break apart ~5% in water, so the predominant form of these species is the neutral molecule. ◦ HF (aq) is shown as HF (aq) Non-electrolytes do not break apart at all. Like weak electrolytes, they are shown as is. H2O (l) is shown as H2O (l) The bottom line: Separate into ions everything with an (aq) except weak acids. This is a solid; it will not break apart. Ca(NO3)2 (aq) + 2 NaF (aq) CaF2 (s) + 2 NaNO3 (aq) These are soluble and will split into ions. The bottom line: Separate into ions everything with an (aq) except weak acids. This subscripts will become a coefficient. It is not part of the polyatomic ion’s formula. Ca(NO3)2 (aq) + 2 NaF (aq) CaF2 (s) + 2 NaNO3 (aq) These subscripts will not be moved because they are part of the polyatomic ion. The bottom line: Separate into ions everything with an (aq) except weak acids. Ca(NO3)2 (aq) + 2 NaF (aq) CaF2 (s) + 2 NaNO3 (aq) The total ionic equation is: Ca2+(aq) + 2 NO3-(aq) + 2 Na+(aq) + 2 F-(aq) CaF2(s) + 2 Na+(aq) + 2 NO3-(aq) Eliminate the spectator ions from the total ionic equation. The spectator ions are the species that look identical in every respect on both sides of the equation. Ca2+(aq) + 2 NO3-(aq) + 2 Na+(aq) + 2 F-(aq) CaF2(s) + 2 Na+(aq) + 2 NO3-(aq) The net ionic equation is: Ca2+(aq)+ 2 F-(aq) CaF2(s) The formula unit equation Ca(NO3)2 (aq) + 2 NaF (aq) CaF2 (s) + 2 NaNO3 (aq) The total ionic equation Ca2+(aq) + 2 NO3-(aq) + 2 Na+(aq) + 2 F-(aq) CaF2(s) + 2 Na+(aq) + 2 NO3-(aq) The net ionic equation Ca2+(aq)+ 2 F-(aq) CaF2(s)






