How do I calculate relative atomic mass
advertisement

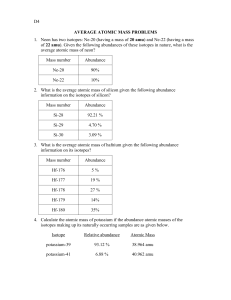
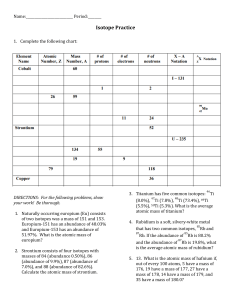
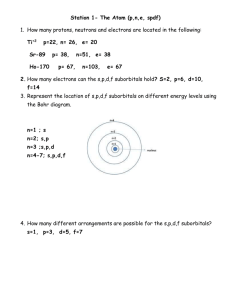
How do I calculate relative atomic mass? Name: and Average Atomic Mass Calculation Example 1.1 BROMINE o bromine consists of 50% 79Br and 50% 81Br, calculate the Ar of bromine. o Ar = [ (50 x 79) + (50 x 81) ] /100 = 80 amu o So the average atomic mass of bromine is 80 amu. o Note the full working shown. Yes, ok, you can do it in your head BUT many students ignore the %'s and just average all the isotopic masses (mass numbers) given, in this case bromine-79 and bromine-81. o and Average Atomic Mass Calculation Example 1.2 CHLORINE o chlorine consists of 75% chlorine-35 and 25% chlorine-37. o Think of the data based on 100 atoms, so 75 have a mass of 35 and 25 atoms have a mass of 37 amu. o The average mass = [ (75 x 35) + (25 x 37) ] / 100 = 35.5 o So the average atomic mass = 35.5 amu o Note: 35Cl and 37Cl are the most common isotopes of chlorine, but, there are tiny percentages of other chlorine isotopes which are usually ignored in high school chemistry. o Please note: The mass number for any isotope is the sum of the protons and neutrons in the nucleus, and is always an integer ( a whole number.) 1. Naturally occurring europium (Eu) consists of two isotopes was a mass of 151 and 153. Europium-151 has an abundance of 48.03% and Europium-153 has an abundance of 51.97%. What is the atomic mass of europium? 2. Calculate the atomic mass of copper if copper-63 is 69.17% abundant and copper-65 is 30.83% abundant. 3. Strontium consists of four isotopes with masses of 84 (abundance 0.50%), 86 (abundance of 9.9%), 87 (abundance of 7.0%), and 88 (abundance of 82.6%). Calculate the atomic mass of strontium. 46 47 48 4. Titanium has five common isotopes: Ti (8.0%), Ti (7.8%), Ti (73.4%), 49Ti (5.5%), 50Ti (5.3%). What is the average atomic mass of titanium?