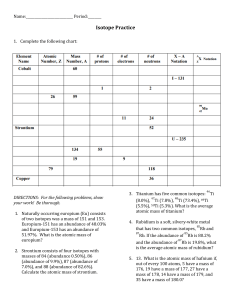
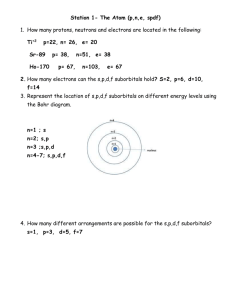
Name: Period: _____ Average Atomic Mass Weighted average of
advertisement
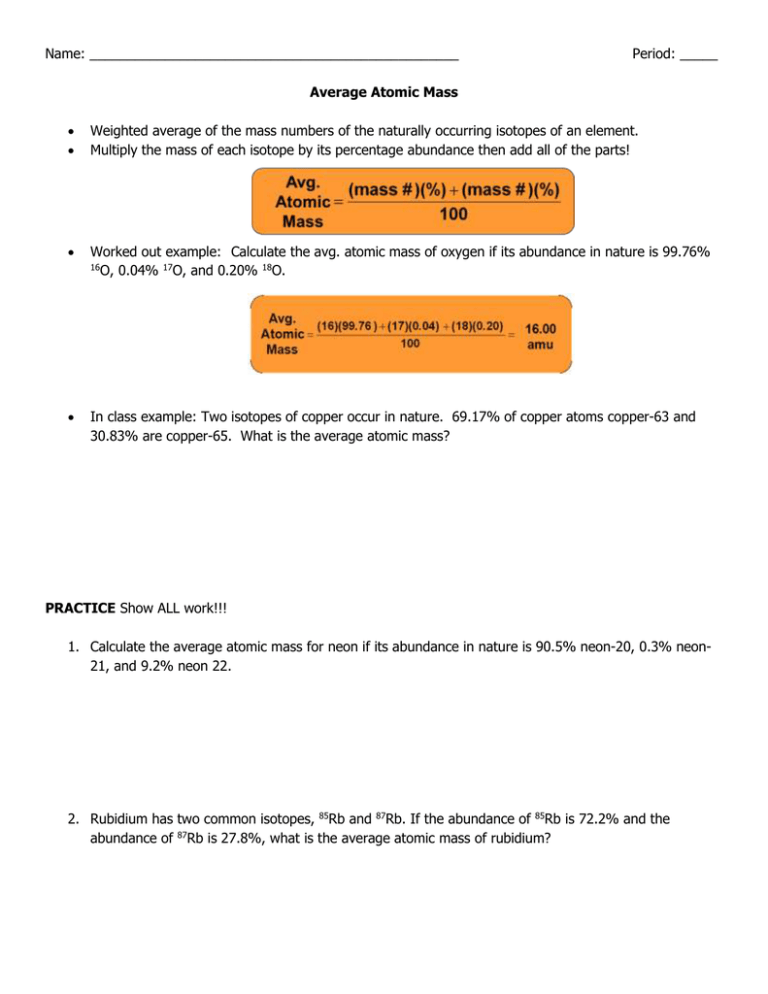
Name: _________________________________________________ Period: _____ Average Atomic Mass Weighted average of the mass numbers of the naturally occurring isotopes of an element. Multiply the mass of each isotope by its percentage abundance then add all of the parts! Worked out example: Calculate the avg. atomic mass of oxygen if its abundance in nature is 99.76% 16 O, 0.04% 17O, and 0.20% 18O. In class example: Two isotopes of copper occur in nature. 69.17% of copper atoms copper-63 and 30.83% are copper-65. What is the average atomic mass? PRACTICE Show ALL work!!! 1. Calculate the average atomic mass for neon if its abundance in nature is 90.5% neon-20, 0.3% neon21, and 9.2% neon 22. 2. Rubidium has two common isotopes, 85Rb and 87Rb. If the abundance of 85Rb is 72.2% and the abundance of 87Rb is 27.8%, what is the average atomic mass of rubidium? 3. Uranium has three common isotopes. If the abundance of 234U is 0.01%, the abundance of 235U is 0.71%, and the abundance of 238U is 99.28%, what is the average atomic mass of uranium? 4. Titanium has five common isotopes: 46Ti (8.0%), 47Ti (7.8%), What is the average atomic mass of titanium? Ti (73.4%),49Ti (5.5%), 48 50 Ti (5.3%). 5. Distinguish between mass number and average atomic mass. Which one is on the periodic table?