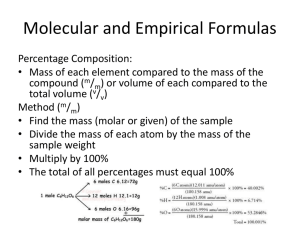
Percentage Composition
advertisement

What is percent composition? Proportion or ratio of each element in a chemical compound Expressed as grams of element per 100 grams of chemical compound Ex. 1 % Composition of MgCO3 Find formula mass Find % of each element in the compound, must all add up to 100% Part Whole Ex. 2 Find the amount (in g) of N in 46.34g of Ammonium nitrate Percent Composition Practice 1. Al2O3 Find the percentages of Al and oxygen in this compound. 2. Ammonium phosphate. What is the percentage of hydrogen in this compound? What is the amount (in grams) of hydrogen? 2 types of chemical formulas 1) Empirical Simplest formula for a molecular compound Lowest whole number ratio of atoms in a compound Many compounds have the same formula Ex. CH2O 2) Molecular Unique to a specific chemical compound Displays the actual # of atoms of each element in a chemical compound “true formula” If we are given just the % composition of each element in a compound—how do we get the molecular formula ???? Pathway to the Molecular Formula 1) Find the empirical formula 1) Use BOTH the empirical formula AND molecular mass to find the molecular formula. Finding the empirical formula--- 1) Assume a 100g sample of chemical compound. Convert % of each element into grams. 2) Convert grams moles for each element 3) Divide each mole amount by the smallest amount. 4) Multiply each number by smallest integer to get whole numbers. Ex. 1 Adipic acid has 49.32% C, 43.84% O, and 6.85% H. Find the empirical formula. Ex. 1 The Sequel Find the molecular formula. Need the empirical formula AND molecular mass. Adipic molecular mass = 146 g/mol Empirical Formula Practice 1. A compound has an empirical formula of C2OH4 and a molar mass of 88 g/mol. Find the molecular formula. 2. Rubbing alcohol contains 60.0%C, 13.4% H, and the remaining mass is from oxygen. What is the empirical formula for rubbing alcohol? 3. Caffeine is found to contain 49.48% C, 5.19% H, 28.85% N, and 16.48% O by mass. The molecular mass of caffeine is 194.2 g/mol. Find the empirical and molecular formula of this compound. Homework For Friday Read pp. 83-89, p. 117 #39, 41, 43 For Thursday Read over lab procedure