Fun With Carbon
advertisement

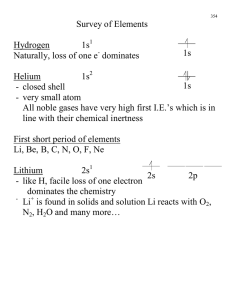
Fun With Carbon CARBON BASIC’S Symbol of carbon is C Atomic number of carbon is 6 Atomic weight of carbon is 12.0107 The electron configuration of carbon is [He]2s22p2 The word origin of carbon is, in Latin carbo, : coal or charcoal. Carbon exists free in nature and has been known since prehistoric time. Descriptions Carbon’s standard state is solid at 298K or 25 degrees Celsius. In solid form Carbon is black as graphite and colorless as a diamond. Carbon is a non-metal. Carbon is found widely in nature. It is found in abundance in suns, stars, comets, and atmospheres of many planets. Allotropes of Carbon Carbon is found in three types in nature, amorphous, graphite, and diamond. Diamond can be found in volcanic vents such as in South Africa. They also can be found on the ocean floor off of the African Coast. . . .other Forms Carbon is found as carbon dioxide in the atmosphere and is found in natural water sources. It can also be found in rocks, and also in coal petroleum and Forms Cont’ Carbon can also occur as graphite naturally. Graphite is found in New York and Texas in the United States, Russia, Greenland, Mexico and India. FACTS Organic chemistry is the study of carbon and its components. Silicon can be used in place of carbon in certain compounds, but unlike carbon it cannot form stable compounds with long chains. Carbons Isotopes In 1961 the International Union of Pure and Applied Chemistry adopted the isotope carbon 12. Carbon 12 can be found on the periodic table. Carbon 14 is used to date minerals such as wood, archeological specimens. Carbon 13 can also be used for isotopic labeling studies because it is also radioactive, but not as stable A new form of carbon, buckminsterfullerene or carbon 60, has be found and is being researched in labs. Uses Of Carbon • Carbon is used in many life processes. • Diamond is used for cutting, drilling, and also as bearings. It is also prized as a gem stone. • Graphite is used for melting metals for rust protection and in pencils. • Amorphous carbon is used for removing tastes and odors. COVALENT BONDING 1) Covalent compounds generally have much lower melting and boiling points than ionic compounds. 2) Covalent compounds are soft and squishy (compared to ionic compounds, anyway). 3) Covalent compounds tend to be more flammable than ionic compounds. 4) Covalent compounds don't conduct electricity in water. 5) Covalent compounds aren't usually very soluble in water. Covalent Notebook Methane, CH4 You will be familiar with drawing methane using dots and crosses diagrams, but it is worth looking at its structure a bit more closely. There is a serious mismatch between this structure and the modern electronic structure of carbon, 1s2 2s2 2px1 2py1. The modern structure shows that there are only 2 unpaired electrons for hydrogens to share with, instead of the 4 which the simple view requires. Methane, CH4 You can see this more readily by using the electrons-in-boxes notation. Only the 2-level electrons are shown. The 1s2 electrons are too deep inside the atom to be involved in bonding. The only electrons directly available for sharing are the 2p electrons. Why then ??? Promotion of an electron When bonds are formed, energy is released and the system becomes more stable. If carbon forms 4 bonds rather than 2, twice as much energy is released and so the resulting molecule becomes even more stable. There is only a small energy gap between the 2s and 2p orbitals, and so it pays the carbon to provide a small amount of energy to promote an electron from the 2s to the empty 2p to give 4 unpaired electrons. The extra energy released when the bonds form more than compensates for the initial input. Hybridization The electrons rearrange themselves again in a process called hybridization. This reorganizes the electrons into four identical hybrid orbitals called sp3 hybrids (because they are made from one s orbital and three p orbitals). You should read "sp3" as "s p three" - not as "s p cubed". Multiple Bonding When two atoms share a single pair of electrons, the bond is referred to as a single bond. Atoms can also share two or three pairs of electrons in the aptly named double and triple bonds. The first bond between two atoms is called the σ (sigma) bond. All subsequent bonds are referred to as π (pi) bonds. In Lewis structures, multiple bonds are depicted by two or three lines between the bonded atoms.