Document
advertisement

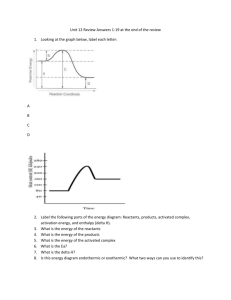
The internal energy of a sample is the sum of all the kinetic and potential energies of all the atoms and molecules in a sample Vaporization Melting Solid Gas Liquid Freezing Condensation Heating Curves Animation A plot of temperature vs. time that represents the process in which energy is added at a constant rate Courtesy Christy Johannesson www.nisd.net/communicationsarts/pages/chem 140 120 Gas - KE Temperature (oC) 100 Boiling - PE 80 60 40 20 0 -20 Liquid - KE Melting - PE -40 -60 -80 Solid - KE -100 Time A plot of temperature vs. time that represents the process in which energy is added at a constant rate • The temperature doesn’t change during a phase change. • If you have a mixture of ice and water, the temperature is 0ºC • At 1 atm, boiling water is 100ºC • You can’t get the temperature higher until it boils Chemical Energy 2 parts of the universe as it relates to a chemical reaction: • System – the reactants and the products • Surroundings – everything else in the universe (such as container, the room, etc.) Law of Conservation of Energy: the total energy of the universe is constant and can neither be created nor destroyed; it can only be transformed. The First Law of Thermodynamics: The total energy content of the universe is constant surroundings system Signs (+/-) will tell you if energy is entering or leaving a system + indicates energy enters a system - indicates energy leaves a system Chemical energy lost by combustion = Energy gained by the surroundings Chemical Energy Two types of processes based on energy flow: • Exothermic – produces energy (heat flows out of the system) • Endothermic – absorbs energy (heat flows into the system) Conservation of Energy in a Chemical Reaction In this example, the energy of the reactants and products decreases, while the energy of the surroundings increases. In every case, however, the total energy does not change. Exothermic Reaction Energy Surroundings mistry, 2004, page 41 System Before reaction Surroundings Reactant System After reaction Product + Energy Conservation of Energy in a Chemical Reaction In this example, the energy of the reactants and products increases, while the energy of the surroundings decreases. In every case, however, the total energy does not change. Surroundings Reactant + Energy Energy Surroundings mistry, 2004, page 41 Endothermic Reaction System System Before reaction After reaction Product Thermochemistry • Every reaction has an energy change associated with it • Energy is stored in bonds between atoms • Making bonds gives energy • Breaking bonds takes energy 20 •To more easily measure and study the energy changes that accompany chemical reactions, chemists have defined a property called enthalpy. •Enthalpy (H) is the heat content of a system at constant pressure. •Although you cannot measure the actual energy or enthalpy of a substance, you can measure the change in enthalpy, which is the heat absorbed or released in a chemical reaction. •The change in enthalpy for a reaction is called the enthalpy (heat) of reaction (∆Hrxn). •You have already learned that a symbol preceded by the Greek letter ∆ means a change in the property. •Thus, ∆Hrxn is the difference between the enthalpy of the substances that exist at the end of the reaction and the enthalpy of the substances present at the start. •Because the reactants are present at the beginning of the reaction and the products are present at the end, ∆Hrxn is defined by this equation. Enthalpy (H) is used to quantify the heat flow into or out of a system in a process that occurs at constant pressure. DH = H (products) – H (reactants) DH = heat given off or absorbed during a reaction at constant pressure Hproducts > Hreactants Hproducts < Hreactants DH > 0 DH < 0 6.4 Energy Change in Chemical Processes reaction Exothermic, heat given off & temperature of water rises Exothermic process: DH < 0 (at constant pressure) Endothermic, heat taken in & temperature of water drops reaction Endothermic process: DH > 0 (at constant pressure) Exothermic process is any process that gives off heat – transfers thermal energy from the system to the surroundings. 2H2 (g) + O2 (g) 2H2O (l) + energy H2O (g) H2O (l) + energy Endothermic process is any process in which heat has to be supplied to the system from the surroundings. energy + 2HgO (s) energy + H2O (s) 2Hg (l) + O2 (g) H2O (l) 6.2 Endothermic Reactions Exothermic Reactions exothermic endothermic exothermic endothermic endothermic Effect of Catalyst on Reaction Rate What is a catalyst? What does it do during a chemical reaction? Catalyst lowers the activation energy for the reaction. No catalyst Energy activation energy for catalyzed reaction reactants products Reaction Progress 140 120 DH = mol x DHfus DH = mol x DHvap Temperature (oC) 100 80 Heat = mass x Dt x Cp, gas 60 40 20 0 Heat = mass x Dt x Cp, liquid -20 -40 -60 -80 Heat = mass x Dt x Cp, solid -100 Time • The heat that is absorbed by one mole of a substance in melting at a constant temperature is the molar heat of fusion DHfus • The heat lost when one mole of a liquid solidifies at a constant temperature is the molar heat of solidification DHsol H2O (s) H2O (l) 33 H2O (l) H2O (g) DHfus = 6.01 kJ/mol DHsol = - 6.01 kJ/mol • The molar heat of vaporization: – Heat needed to change one mol of a liquid to gas DHvap • The molar heat of condensation: – Heat needed to change one mol of a gas to liquid DHcon H2O (l) H2O (g) 34 H2O (g) H2O (l) DHvap = 40.7 kJ/mol DHcon = - 40.7 kJ/mol • The heat that is released or absorbed in a chemical reaction is equivalent to DH C + O2(g) C + O2(g) CO2(g) +394 kJ CO2(g) DH = -394 kJ • In thermochemical equation it is important to say what state H2O(g) H2O(l) H2(g) + ½ O2 (g) H2(g) + ½ O2 (g) DH = 241.8 kJ DH = 285.8 kJ Difference = 44.0 kJ 35 “In going from a particular set of reactants to a particular set of products, the change in enthalpy is the same whether the reaction takes place in one step or a series of steps.” • The change in heat that accompanies the formation of a mole of a compound from its elements at standard conditions • Standard conditions 25°C and 1 atm. • Symbol is DH◦f The standard heat of formation of an element at its most stable form is 0 This 38 includes the diatomics • There are tables of heats of formations (pg. 316) • For most compounds it is negative – Because you are making bonds – Making bonds is exothermic • The heat of a reaction can be calculated by subtracting the heats of formation of the reactants from the products DH = DH◦f (products) - DH◦f (reactants) 39 1. If a reaction is reversed, the sign of ∆H must be reversed as well. – because the sign tells us the direction of heat flow as constant P 2. The magnitude of ∆H is directly proportional to quantities of reactants and products in reaction. If coefficients are multiplied by an integer, the ∆H must be multiplied in the same way. – because ∆H is an extensive property If H2(g) + 1/2 O2(g) H2O(l) D H=-285.5 kJ/mol then H2O(l) H2(g) + 1/2 O2(g) D H =+285.5 kJ/mol If you turn an equation around, you change the sign 2 H2O(l) 2 H2(g) + O2(g) D H =+571.0 kJ/mol If you multiply the equation by a number, you multiply the heat by that number. – Twice the moles, twice the heat 41 • You make the products, so you need their heats of formation • You “unmake” the reactants so you have to subtract their heats. ΔHreaction Σn pΔHf(products) Σn r ΔHf(reactant s) https://www.youtube.com/watch?v=_NLAgSnqNOE&noredirect=1 42 Calculate the heat of combustion of methane, CH4 CH4(g) + 2 O2(g) CO2(g) + 2 H2O(g) DH◦f CH4 (g) = -74.86 kJ/mol DH◦f O2(g) = 0 kJ/mol DH◦f CO2(g) = -393.5 kJ/mol pg. 316 DH◦ fH2O(g) = - 241.8 kJ/mol H2 (g) + ½ O2 (g) H2O(g) 2x(- 241.8)= - 483.6kJ/mol Step #1: since 2 moles of water are produced by each mole of methane, we multiply the DH◦ f. of water by 2. 43 Calculate the heat of combustion of methane, CH4 CH4(g) + 2 O2(g) CO2(g) + 2 H2O(g) DH◦f CH4 (g) = +74.86 kJ/mol DH◦f O2(g) = 0 kJ/mol DH◦f CO2(g) = -393.5 kJ/mol pg. 316 DH◦ fH2O(g) = -483.6 kJ/mol DH◦f = [-393.5 kJ/mol + (-483.6 kJ/mol)]- [-74.86 kJ/mol + (0 kJ/mol )] DH◦f = [-393.5 -483.6] + 74.86 = -877.1 + 74.86 = -802.2 kJ/mol Step #2: sum up all the DH◦ f. : DHrxn = DHf(products) - D Hf(reactants) 44 Specific Heat capacity (J/oC) = heat supplied (J) temperature (oC) Specific Heat Capacity = heat required to raise the temperature of 1 gram of a substance object by 1 oC Affected by −What the substance is −Mass of the object The amount of heat absorbed or released during a physical or chemical change can be measured… …usually by the change in temperature of a known quantity of water 1 calorie is the heat required to raise the temperature of 1 gram of water by 1 C 1 BTU is the heat required to raise the temperature of 1 pound of water by 1 F – A device used to experimentally determine the amount of heat released or absorbed during a physical or chemical change heat gained = heat lost Most common units of energy 1. S unit of energy is the joule (J), defined as 1 (kilogram•meter2)/second2, energy is also expressed in kilojoules (1 kJ = 103J). 2. Non-S unit of energy is the calorie. One cal = 4.184 J or 1J = 0.2390 cal. Units of energy are the same, regardless of the form of energy The amount of heat required to raise the temperature of one gram of substance by one degree Celsius. Substance Specific Heat (J/g·K) Water (liquid) 4.18 Ethanol (liquid) 2.44 Water (solid) 2.06 Water (vapor) 1.87 Aluminum (solid) 0.897 Carbon (graphite,solid) 0.709 Iron (solid) 0.449 Copper (solid) 0.385 Mercury (liquid) 0.140 Lead (solid) 0.129 Gold (solid) 0.129 Page 296 • The higher the specific heat the more energy it takes to change its temperature. • Pizza burning the roof of your mouth • The same amount of heat is released when an object cools down 50 Q = m. C . DT Q = Heat lost or gained ( J) C = Specific Heat (J/ ºC.g) DT = Temperature change = Tf – Ti (ºC) Change in energy = mass * specific heat * change in temp. How much heat is need to raise 5 g of water 10 ̊C? (Water’s specific heat is 4.18 J/(̊C.g) Known m= 5 Kg DT = 10 ̊C C = 4.18 J/(g- ̊C) Unknown heat needed? 2. Q = m * C5g * *DT C * DT 4.18 J/(g- ̊C) * DT 10 ̊C 3. Q = 209 J 1. Q = m * C * DT 28,875 J of energy are added to a 5-kg piece of copper that has an initial temperature of 293 K. What will be the final temperature of this piece of copper? (Copper’s specific heat:385 J/(kg-K)) Known m= 5 Kg Ti = 293 K C = 385 J/(g- C) Unknown Final temperature? 1. Q = m * C * DT 2. 28,875 J = 5m kg ** C 385 C ** DT J/(kg-K) DT * DT 3. DT = 15 K 293 K + 15 K = 308 K 1. Q = m * C * DT 140 120 DH = mol x DHfus DH = mol x DHvap Temperature (oC) 100 80 Heat = mass x Dt x Cp, gas 60 40 20 0 Heat = mass x Dt x Cp, liquid -20 -40 -60 -80 Heat = mass x Dt x Cp, solid -100 Time Choose all that apply... C(s) + 2 S(g) CS2(l) DH = 89.3 kJ Which of the following are true? A) This reaction is exothermic B) It could also be written C(s) + 2 S(g) + 89.3 kJ CS2(l) C) The products have higher energy than the reactants D) It would make the water in the calorimeter colder