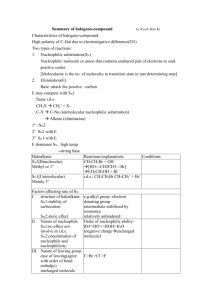
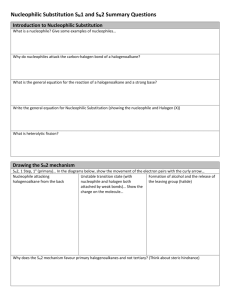
20.1 Nucleophilic Substitution Reactions
advertisement

WARM-UP 12/14 1. Use wedges to represent the an S N2 reaction. What is happening to the bonded H’s around the Carbon? SN2 reactions are stereospecific and have an inversion of configuration. WARM-UP 12/15 • Show reaction pathway for: a) but-2-ene to butanone b) ethane to ethanol IA STUFF HOW WOULD YOU DESCRIBE BENZENE? • Unsaturated, aromatic hydrocarbon EVIDENCE FOR BENZENE’S STRUCTURE • X-ray diffraction showed equal bond lengths • Bond lengths = intermediate between single and double (bond order = 1.5) • Electrostatic potential mapping (all carbons have equal electrical density) MORE EVIDENCE FOR BENZENE • Does not decolorize bromine water • Incredibly stable because of delocalization WARM-UP 1/4 WELCOME BACK! • Use diagrams to explain why S N1 reactions primarily occur with tertiary haloalkanes and SN2 reactions primarily occur with primary haloalkanes. ANSWERS TO WARM-UP 1. Tertiary carbocations are relatively stable. -inductive effect of alkyl groups -reduces charge on central carbon atom, stabilizing carbocation intermediate 2. Change in geometry from tetrahedral (109⁰) to trigonal planar (120⁰) in SN1 intermediate. -stabilizes carbocation by reducing repulsion 3. In SN1 reactions the alkyl groups around carbocation hinders attack of nucleophile IA STUFF • Final Draft-turnitin.com, hard copy and hapara folder please • Due NO LATER than 11:59 pm, Friday January 29 th. • MUCH more CHEMISTRY in your background/introduction and conclusion. • The “why” and “what” behind your procedure. • Read your procedure from an outsider’s perspective. • MUCH more detail needed (and materials list) • Make sure that your data is SPECIFIC to answering your RESEARCH QUESTION!!!!! • CITATIONS!!!! HALOGENATED AROMATIC COMPOUNDS • Much less reactive than other halogenalkanes. • Why is this? (look back at answer) 1. C-Cl bond much stronger because lone pairs on Cl are part of “delocalization” ring around benzene 2. Very little room for nucleophile to attack carbon 3. Partial charge on carbon reduced because of delocalization effect. WARM-UP 1/5 • Describe the differences in the carbon- halogen bonds of 2-bromohexane and 2-chlorohexane. • How might these differences affect the rate of a nucleophilic substitution reaction? 20.2 NUCLEOPHILIC SUBSTITUTION REACTIONS (HL) • Factors affecting reaction rate of SN1 and SN2 reactions NUCLEOPHILE ON REACTION RATE • Why this trend? • Explain why OH- is a better nucleophile than water Lone pairs of e- (and negative charge) = stronger Lower electronegativity of atom with lone pairs = stronger RATES DEPENDING UPON HALOGENALKANES • 2 factors: • Polarity of carbon – halogen bond • Predict reaction rate of I alkane to be less than with Cl as halogen. • Why? • Strength of carbon-halogen bond • Decreases from chlorine to iodine • Expect weaker bond = easier to break = faster reaction • Predict reaction rate of Cl alkane to be slower than I alkane. SO WHICH IS IT- POLARITY OR BOND STRENGTH? • Bond strength • Rate of hydrolysis for iodoalkanes greater than for chloroalkanes. COMPARING SN1 AND SN2 REACTIONS Because Ea of both steps in SN1 reaction are lower than Ea of SN2 reaction. -transition state of SN2 is very unstable so at a high potential energy SN1 SN2 NUCLEOPHILIC SUBSTITUTION WITH AMMONIA • Products? • Bromoethane ? • Can have multiple substitutions: QUATERNARY AMMONIUM SALT • After four nucleophilic substitutions reactions: NUCLEOPHILIC SUBSTITUTION WITH CYANIDE • Products? • Bromoethane ? REDUCTION OF NITRILES • Similar to hydrogenation of alkenes • Need nickel catalyst! • Hydrogen is added to break triple bonds and form primary amines. CHOICE OF SOLVENT • SN1 = protic, polar solvents • Methanol, water, ammonia, methanoic acid, hydrogen fluoride. • How will these solvents interact with aqueous NaOH? PROTIC POLAR SOLVENTS • Will Hydrogen bond with the OH • Solvation • Why is this important in S N1 reactions, but would seriously inhibit SN2 reaction mechanism? SN2 REACTIONS NEED APROTIC, POLAR SOLVENTS • Ethyl ethanoate, propanone • No hydrogen bonding so leave nucleophile “naked” and therefore more effective. ELECTROPHILIC ADDITION REACTIONS • Outline what is meant by the term “electrophilic addition reactions” • Reactants? • Alkenes (why?) • What are electrophiles? • NO2+ , CH3+ CURLY ARROW MECHANISM • Bute-1-ene and hydrogen iodide MARKINOV’S RULE • Hydrogen atom will preferentially bond to carbon atom of alkene with largest number of hydrogen • Carbocation stability • Most stable with most alkyl groups • Tertiary carbocation > secondary > primary INTERHALOGEN ADDITION REACTIONS • I-Br • Br-Cl • Which attacks first? BOOK PROBLEMS-PG. 459-460 • #4, 9 (not ‘a’)