Diapositiva 1
advertisement

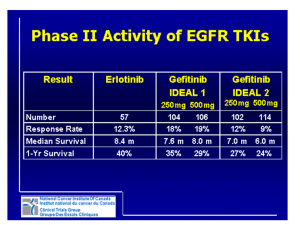
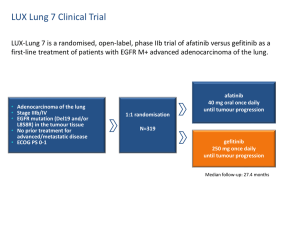
Advantages and disavantages of irreversible EGFR-TKI Lucio Crinò, MD Medical Oncology Department University Hospital Perugia, Italy Evolution of Knowledge in NSCLC From Histology to Molecular Classification Li, et al. J Clin Oncol 2013 EGFR mutations in NSCLC Second generation EGFR TKIs • The second-generation of EGFR TKIs has the advantage of forming covalent, irreversible bonds with the target, which should increase their effectiveness through a prolonged inhibition of EGFR signaling • Furthermore, the prolonged and irreversible inhibition of the receptor has the potential for further improvement in response to treatment over the first-generation TKIs such as erlotinib and gefitinib • To date, irreversible oral TKIs that target simultaneously multiple members of the EGFR family are currently in clinical development for NSCLC, including afatinib, dacomitinib and neratinib. • Preclinical studies showed that irreversible TKIs killed cells with acquired resistance to first-generation TKIs. Becker K et al., World J Clin Oncol. 2014 Afatinib Is the First Irreversible ErbB Family Blocker • Afatinib selectively, covalently binds and irreversibly blocks ErbB family receptors EGFR, HER2, and ErbB4 • Afatinib prevents ligand-dependent ErbB3 phosphorylation in preclinical studies Solca et al. J Pharmacol Exp Ther. 2012;343:342. Li et al. Oncogene. 2008;27:4702; Köhler J et al. Onkologie 2013;36:510-518 Dacominitinb mechanism of action • Dacomitinib is an irreversible panHER tyrosine kinase inhibitor with activity against EGFR, HER2 and HER4. • Preclinical activity against – EGFR sensitising mutations – EGFR T790M – wild-type HER2 – mutant HER2 Peters S., Cancer Treatment Review, 2014 Pharmacokinetics of afatinib and dacomitinib • Afatinib undergoes minimal metabolism by the cytochrome P450 system but is a substrate of p-glycoprotein. P-glycoprotein inducers (rifampicin,carbamazepine, phenytoin, phenobarbital, St. John’s Wort ) may therefore lower systemic drug concentrations of afatinib. • Coadministration of a P-glycoprotein inhibitor (ritonavir, cyclosporine, ketoconazole, erythromycin, verapamil, and others) can increase afatinib exposure. • As oral drugs, gastric contents and pH may also impact bioavailability. Afatinib absorption is reduced when taken with a high fat meal whereas erlotinib absorption is increased and patients are directed to take both medications on an empty stomach USA: Boehringer Ingelheim Pharmaceuticals, Inc Ridgefield 2013. Potency and inhibition of cell proliferation of oral EGFR tyrosine kinase inhibitors Peters S et al. Cancer Treatment Reviews (2014) Studies of EGFR TKIs versus chemotherapy as first-line therapy in EGFR Act Mut+ NSCLC Study Median Median OS in PFS in TKI Median PFS in TKI Median OS in arm chemotherapy arm chemotherapy EGFR TKI (months) arm (months) (months) arm (months) OPTIMAL Erlotinib 13.1 4.6 22.7 28.9 First Signal Gefitinib 8.4 6.3 27.2 25.6 IPASS Gefitinib 9.5 6.3 21.6 21.9 WJTOG 3405 Gefitinib 8.4 5.3 36 39 NEJSG 002 Gefitinib 10.8 5.4 27.7 26.6 EURTAC Erlotinib 10.4 5.2 19.3 19.5 LUX-3 Afatinib 11.1 6.7 28.1 28.2 5.6 Not reported, Not reported, immature LUX-6 Afatinib 11.0 LUX-Lung 3 and 6: OS in common mutations Presented By James Yang at 2014 ASCO Annual Meeting Combined OS analysis: common mutations (n=631) Presented By James Yang at 2014 ASCO Annual Meeting Combined OS analysis in common mutations: subgroups Presented By James Yang at 2014 ASCO Annual Meeting Combined OS analysis: mutation categories Presented By James Yang at 2014 ASCO Annual Meeting LUX-Lung 3 and LUX-Lung 6: Summary of Adverse Events % of Patients LUX-Lung 31,2 LUX-Lung 63,4 Afatinib (n=229) Pem/Cis (n=111) Afatinib (n=239) Gem/Cis (n=113) Drug-related AEs 100 96 99 99 Drug-related AE grade ≥3 49 48 36 60 Drug-related AEs leading to discontinuation 8a,b 12 6c 40 0 0 2.1 (5 pts) 0 1.3 (3 pts) 0 0 0 14 14 5 7 1.7 (4 pts)d 0 0.4 (1 pt)e 0.9 (1 pt)e Discontinuation due to rash Discontinuation due to diarrhoea Drug-related SAE Related SAE leading to death aIncludes 3 patients (1%) who discontinued due to diarrhoea, no discontinuations for rash. 3 patients (1%) with ILD-like events (1 grade 1, 1 grade 3; 1 grade 5). cIncluding 1 patient with ILD. dPreferred terms: dyspnoea, sepsis, ARDS, death (unknown cause). eSudden death (afatinib) and cardiac failure (Gem/Cis). SAE = serious adverse event; ILD = interstitial lung disease; ARDS = acute respiratory distress syndrome. bIncludes 1. Sequist et al. J Clin Oncol. 2013;31:3327; 2.Yang ASCO 2013 ; 3. Wu et al. Lancet Oncol. 2014;15:213. 4. Wu YL. et al. 2013 ASCO Annual Meeting. Oral Presentation Most Frequent Treatment-Related Adverse Events (>20% Difference Between Treatment Arms) LUX-Lung 31, / LUX-Lung 62, % Afatinib LL3 (n=229) / LL6 (n =239 ) aGrouped Pem/Cis (n=111) / Gem/Cis (n=113) All Grades Grade 3 Grade 4 All Grades Grade 3 Grade 4 Rash/acnea 89.1 / 80.8 16.2 / 14.2 0.0 / 0.4 6.3 / 8.8 0 0 Diarrhoea 95.2 / 88.3 14.4 / 5.4 0 15.3 / 10.6 0 0 Paronychia/nail effecta 56.8 / 32.6 11.4 / 0.0 0 0/0 0 0 Stomatitis/mucositisa 72.1 / 51.9 8.37 / 5.4 0.4 / 0.0 15.3 / 5.3 0.9 / 0.0 0 Decreased appetite 20.5 / 10.0 3.1 / 1.3 0 53.2 / 40.7 2.7 / 1.8 0 Vomiting 17.0 / 9.6 3.1 / 0.8 0 42.3 / 80.5 2.7 / 15.9 0.0 / 3.5 Fatiguea 17.5 / 10.0 1.3 / 0.4 0 46.8 / 36.3 12.6 / 0.9 0 Nausea 17.9 / 7.5 0.9 / 0.0 0 65.8 / 75.2 3.6 / 7.1 0.0 /0.9 Dry skin 29.3 / NA 0.4 / NA 0 1.8 / NA 0 0 Pruritus 18.8 / 10.9 0.4 / 0.4 0 0.9 / 0.0 0 0 Neutropenia 0.9 / 2.1 0.4 / 0.4 0 31.5 / 54.0 15.3 / 17.7 2.7 / 8.8 Anemia 3.1 / 5.4 0.4 / 0.4 0 27.9 / 27.4 4.5 / 7.1 1.8 / 1.8 Leukopenia 1.7 / 3.3 0.04 / 0.4 0 18.9 / 51.3 8.1 / 13.3 0.0 / 1.8 ALT increase 7.4 / 20.1 0.0 / 1.7 0 2.7 / 15.9 0.0 / 1.8 0.0 / 0.9 AST increase 5.2 / 15.1 0.0 / 0.4 0 1.8 / 10.6 0.0 / 1.8 0 term for closely related AEs. ALT = alanine aminotransferase; AST = aspartate aminotransferase. 1. Sequist et al. J Clin Oncol. 2013;31:3327 2. Wu et al. Lancet Oncol. 2014;15:213. 15 Is response rate improved with irreversible EGFR-TKIs? Comparison of best reported phase II results for EGFR TKIs in patients with EGFR-Mutant lung cancers (Exon 19 and Exon 21) Entered, n CR+PR Rate, % Median PFS, months Median OS, months 46 74 17 NR Afatinib1 129a 66 15b 32–39 Erlotinib2 33 70 14 31 Gefitinib3 27 59 Agent Dacomitinib Weighted pooled analysis median PFS in patients with EGFR-mutant lung cancers4 Erlotinib (95% CI) 365c 13.2 (12.0–14.7) Gefitinib (95% CI) 1069d 9.8 (9.2–10.4) a51 treated first-line; bmedian PFS: 12 months on blind review; c12 studies; d39 studies 1Yang JC, et al. Lancet Oncol 2012;3: 539–48. PA, et al. J Clin Oncol 2012;epub 30April. 3Sequist LV, et al. J Clin Oncol 2008;26: 2442–9 4Paz-Ares L, et al. J Cell Mol Med 2010;14:51–69. 2Janne NR, not reached; OS, overall survival Is PFS improved with irreversible EGFR-TKIs? Indirect comparison in patients with classical EGFR mutations in first-line Erlotinib: EURTAC Gefitinib:IPASS HR 0.37, p<0.0001 HR 0.48, p<0.0001 1.0 0.9 0.8 0.7 0.6 0.5 0.4 0.3 0.2 0.1 Gefitinib: median 9.5 months Erlotinib: median 9.7 months 0 0 4 8 12 16 20 24 Progression-free survival (probability) Dacomitinib:phase II Afatinib:phase III 1.0 0.9 HR 0.47, p<0.0001 0.8 0.7 Median 16.8 months 0.6 0.5 0.4 0.3 0.2 0.1 0 0 5 10 15 20 25 30 Afatinib: median 13.6 months Indirect comparison of reversible vs. irreversible EGFR-TKIs Erlotinib Gefitinib Afatinib NEJSG 002 n=114 IPASS n=607 First-SIGNAL n=159 WJTOG3405 n=87 OPTIMAL n=83 CALGB30406 n=81 Rash 71.0 (5.3) 66.2 (3.1) 72.3 (1.3) 74 (2) 73.5 (2.4) NR (7.4) 37 (16.2) Diarrhoea 34.2 (0.9) 46.6 (3.8) NR 47(1) 25.3 (1.2) NR (4.9) 33 (14.4) Fatigue 10.5 (2.6) NR 28.3 (0.6) 34 (2) 4.8 (0) NR (1.2) 3 (1.3) Anorexia NR 21.9 (1.5) 44.7 (0) NR NR NR 7 (3.1) Stomatitis 9.6 (0) 17.0 (0.2) NR 19 (0) 13.3 (1.2) NR 20 (8.7) Paronychia NR 13.5 (0.3) NR 28 (1) 3.6 (0) NR 26 (11.4) 6.1 (0.9) 12.9 (0.2) NR NR NR NR 7 (3.1) Vomiting LUX-3 n=229 LUX-Lung 7 – Trial Design LUX-Lung 7 A multicentre, randomized, open-label phase IIb of afatinib vs. gefitinib as first-line treatment for patients with advanced and metastatic non-small cell lung cancer (NSCLC) harbouring EGFR activating mutations Patients with: • Adenocarcinoma of the lung • Presence of activating EGFR mutations in the tumor tissue either by local lab or • Stage IIIB/IV • No prior treatment with chemotherapy for advanced/metastatic disease • No prior treatment with EGFR-inhibitors • ECOG 0 or 1 by central lab Randomize 1:1 Oral afatinib 40 mg once daily Oral gefitinib 250 mg once daily Primary endpoint: progression-free survival (PFS) NCT01466660 Archer 1050 – Trial Design Archer 1050 A multicentre, randomized, open-label phase III, clinicas study comparing dacominitinib vs. gefitinib as first-line treatment for patients with advanced and metastatic non-small cell lung cancer (NSCLC) harbouring EGFR activating mutations Patients with: • Adenocarcinoma of the lung • Presence of activating EGFR mutations in the tumor tissue either by local lab or by central lab. EGFR mutation status •Stage IIIB/IV • No prior treatment with chemotherapy for advanced/metastatic disease • No prior treatment with EGFR-inhibitors • ECOG 0 or 1 Randomize 1:1 Oral daconitinib 45 mg once daily Oral gefitinib 250 mg once daily Primary endpoint: progression-free survival (PFS) EGFR TKIs and EGFR mutated NSCLC:<br />major mechanisms of resistance to EGFR TKIs (2) Presented By Daniel Costa at 2014 ASCO Annual Meeting Modest efficacy of irreversible EGFR-TKIs Against “de novo” and “acquired” T790M LUX LUNG 1: RR=7% LUX-LUNG 2-3-6 trials LUX LUNG 4: RR=8% T790M Response rate (%) 14.3 PFS (months) 2.9 OS (months) 14.9 Neratinib RR=0% in T790M+ Conclusions • No relevant differences in clinical activity between first and second generation TKIs • Better preclinical data of irreversible TKIs do not translate in clinical practice • Similar results in uncommon EGFR mutations • More favorable Gefitinib Erlotinib toxicity profile • Improved efficacy of Afatinib in exon 19 deletion? • Face to Face comparative trials ongoing