LUX-Lung 7 Trial: Afatinib vs Gefitinib in Lung Cancer
advertisement
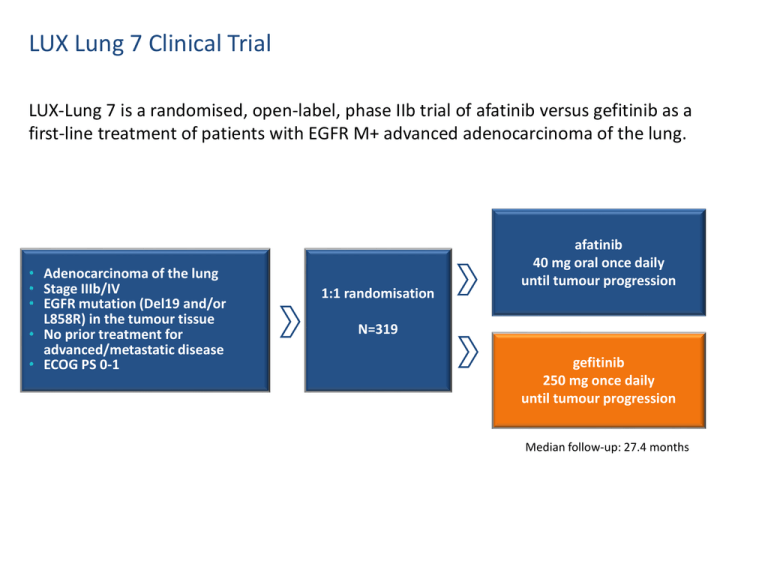
LUX Lung 7 Clinical Trial LUX-Lung 7 is a randomised, open-label, phase IIb trial of afatinib versus gefitinib as a first-line treatment of patients with EGFR M+ advanced adenocarcinoma of the lung. • Adenocarcinoma of the lung • Stage IIIb/IV • EGFR mutation (Del19 and/or L858R) in the tumour tissue • No prior treatment for advanced/metastatic disease • ECOG PS 0-1 1:1 randomisation afatinib 40 mg oral once daily until tumour progression N=319 gefitinib 250 mg once daily until tumour progression Median follow-up: 27.4 months LUX Lung 7 Study Endpoints Primary endpoints • Progression-free survival (PFS) by independent review • Time to treatment failure (TTF): the time from randomisation to discontinuation for any reason, allowing continuation of treatment if physicians consider patients to be receiving clinical benefit • Overall survival (OS) Secondary endpoints included • Objective response rate (ORR) • Time to and duration of response • Duration of disease control • Tumour shrinkage • Health related quality of life (HRQoL), as reported by EQ-5D patient questionnaires LUX Lung 7: 64 sites in 14 countries Recruitment: Dec 2011-Aug 2013 Canada 31 Germany 14 Spain 25 France 34 Norway 3 Sweden 14 UK 7 Ireland 9 Australia China 48 Hong Kong 6 Taiwan 22 Korea 56 Singapore 27 19 3 Patient demographics and characteristics were well balanced between the 2 treatment arms Afatinib 160, N (%) Gefitinib 159, N (%) Total 319, N (%) 63 (30-86) 63 (32-89) 63 (30-89) Female 91 (56.9) 106 (66.7) 197(61.8) Male 69 (43.1) 53 (33.3) 122 (38.2) Asian 94 (58.8) 88 (55.3) 182 (57.1) Non-Asian* 66 (41.2) 71 (44.7) 137 (42.9) Brain mets 26 (16.3) 25 (15.7) 51 (16.0) Never smoker 106 (66.3) 106 (66.7) 212 (66.5) 0 51 (31.9) 47 (29.6) 98 (30.7) 1 109 (68.1) 112 (70.4) 221 (69.3) IIIB 8 (5.0) 3 (1.9) 11 (3.5) IV 152 (95.0) 156 ( 98.1) 308 (96.6) Del19# 93 (58.1) 93 (58.4) 186 (58.3) L858R 67 (41.9) 66 (41.6) 133 (41.7) Age, median yrs (min-max) Gender Race Baseline ECOG NSCLC stage EGFR mutation LUX-LUNG 7 Efficacy Afatinib demonstrated a 27% reduction in relative risk of death or progression compared to gefitinib Progression-free survival by independent review (primary endpoint) Median (months) 11.0 10.9 1.0 Afatinib® (n=160) Gefitinib (n=159) Estimated PFS probability 0.8 Hazard ratio 0.73 (95% CI, 0.57-0.95) 0.6 P=0.0165 27% vs 15% 18% 0.4 vs 8% 0.2 0.0 0 3 6 9 12 15 18 21 24 27 30 33 36 39 42 Time of progression free survival (months) • Patients twice as likely to be alive and progression free at 2 years with afatinib vs gefitinib respectively) 6 Park K et al. Abstract LBA2 and oral presentation. European Society for Medical Oncology (ESMO) ASIA, Singapore, 20th December 2015. (18% vs 8%, Afatinib – PFS benefit consistent across subgroups, including EGFR mutation type Progression-free survival by independent review (primary endpoint) prespecified subgroups Factors Number of patients Total Hazard ratio (95% Cl) 319 0.732 (0.566, 0.947) 133 186 0.708 (0.475, 1.055) 0.764 (0.549, 1.063) 268 51 0.739 (0.560, 0.976) 0.764(0.405, 1.439) 98 221 0.892 (0.542, 1.469) 0.705 (0.524, 0.948) 122 197 0.876 (0.585, 1.312) 0.653 (0.469, 0.910) 177 142 0.681 (0.479, 0.968) 0.845 (0.585, 1.221) 137 182 0.717 (0.487, 1.056) 0.756 (0.539, 1.060) 212 40 67 0.801 (0.584, 1.097) 1.094 (0.559, 2.140) 0.477 (0.270, 0.845) EGFR mutation L858R Del19 Brain métastasés Absent Present Baseline ECOG score 0 1 Gender Maie Female Age group <65 years >65 years Race Non-Asian Asian Smoking hïstory Never smoked <15 pack years + stopped >1 year before Other current or ex-smokers 1/16 Favours afatinib 7 Park K et al. Abstract LBA2 and oral presentation. European Society for Medical Oncology (ESMO) ASIA, Singapore, 20th December 2015. 1/4 1 4 Favours gefitinib 16 Patients remained on treatment significantly longer with afatinib than with gefitinib Time to treatment failure (primary endpoint) Median (months) 13.7 11.5 Estimated probability of being free of treatment failure 1.0 Afatinib® (n=160) Gefitinib (n=159) 0.8 Hazard ratio 0.73 (95% CI, 0.58-0.92) 0.6 P=0.0073 0.4 0.2 0.0 0 3 6 9 12 15 18 21 24 27 30 33 36 39 Time to treatment failure (months) • 27% significant reduction in relative risk of treatment failure vs gefitinib (P=0.0073) • Overall survival data not yet mature TTF is the time from randomisation to discontinuation for any reason, allowing continuation of treatment if physicians consider patients to be receiving clinical benefit 8 Park K et al. Abstract LBA2 and oral presentation. European Society for Medical Oncology (ESMO) ASIA, Singapore, 20th December 2015. 42 Afatinib demonstrated significantly improved response rates vs gefitinib Objective response by independent review (secondary endpoint) P=0.0083 Objective response rate (%) 80% 70% 56% 60% 40% 20% 0% Afatinib Gefitinib • Improved objective response and disease control rates vs gefitinib (ORR: 70% vs 56%, P=0.0083; DCR: 91.3% vs 87.4%) • Longer duration of response vs gefitinib (10.1 vs 8.4 months, respectively) 9 Park K et al. Abstract LBA2 and oral presentation. European Society for Medical Oncology (ESMO) ASIA, Singapore, 20th December 2015. Greater tumour shrinkage in all mutations with afatinib versus gefitinib Tumour shrinkage by independent review (secondary endpoint) Maximum decrease from baseline (%) 40 20 0 -20 -40 -60 Afatinib -80 Gefitinib -100 Based on maximum percentage decrease from baseline in the sum of target lesion diameters. Park K et al. Abstract LBA2 and oral presentation. European Society for Medical Oncology (ESMO) ASIA, Singapore, 20th December 2015. All mutations Greater tumour shrinkage in Del19 mutation patients with afatinib versus gefitinib 40 20 Afatinib 0 -20 -40 -60 -80 -100 Maximum decrease from baseline (%) Maximum decrease from baseline (%) Tumour shrinkage by independent review (secondary endpoint) Del19 mutations 40 20 Gefitinib 0 -20 -40 -60 -80 -100 ≥20% increase >0 - <30% decrease ≥0 - <20% increase ≥30 - <50% decrease Based on maximum 11 percentage decrease from baseline in the sum of target lesion diameters. Park K et al. Abstract LBA2 and oral presentation. European Society for Medical Oncology (ESMO) ASIA, Singapore, 20th December 2015. ≥50% decrease Greater tumour shrinkage in L858R mutation patients with afatinib versus gefitinib 40 20 Afatinib 0 -20 -40 -60 -80 -100 Maximum decrease from baseline (%) Maximum decrease from baseline (%) Tumour shrinkage by independent review (secondary endpoint) L858R mutations 40 20 Gefitinib 0 -20 -40 -60 -80 -100 ≥20% increase >0 - <30% decrease ≥0 - <20% increase ≥30 - <50% decrease Based on maximum 12 percentage decrease from baseline in the sum of target lesion diameters. Park K et al. Abstract LBA2 and oral presentation. European Society for Medical Oncology (ESMO) ASIA, Singapore, 20th December 2015. ≥50% decrease LUX-LUNG 7 Adverse events 13 Pattern of AEs consistent with the known profiles of both agents • The most common AEs were predictable and generally manageable through supportive care and dose reduction AE=adverse event; ILD=interstitial lung disease. Park K et al. Abstract LBA2 and oral presentation. European Society for Medical Oncology (ESMO) ASIA, Singapore, 20th December 2015. AE-related discontinuation Objective response rate (%) • Equally low rates of AE-related discontinuation (6.3% vs 6.3%) with differing causes; skin-related, diarrhoea and fatigue for GIOTRIF®, liver enzyme elevation and ILD for gefitinib 15% 10% 6.3% 6.3% Afatinib Gefitinib 5% 0% 14 Pattern of AEs consistent with the known profiles of both agents Afatinib Gefitinib Related Aes occuring in >10% patients, n(%) All Grades Grade 3 All Grades Grade 3 Diarrhoea 144 (90.0) 19 (11.9) 97 (61.0) 2 (1.3) Rash/Acne* 142 (88.8) 15 (9.4) 129 (81.1) 5 (3.1) Stomatitis* 103 (64.4) 7 (4.4) 38 (23.9) Paronychia* 89 (55.6) 3 (1.9) 27 (17.0) Dry skin 52 (32.5) 59 (37.1) Pruritus 37 (23.1) 36 (22.6) Fatigue* 33 (20.6) 9 (5.6) 23 (14.5) Decreased appetite 26 (16.3) 1 (0.6) 19 (11.9) Nausea 26 (16.3) 2 (1.3) 22 (13.8) Alopecia 17 (10.6) 24 (15.1) Vomiting 17 (10.6) 6 (3.8) 1 (0.6) ALT increase 15 (9.4) 38 (23.9) 12 (7.5) AST increase 10 (6.3) 33 (20.8) 4 (2.5) *Preferred terms of AEs. 4 cases of ILD with gefitinib, 3 of them ≥ grade 3, no case of ILD with GIOTRIFR. 1 case of grade 4 diarrhoea with GIOTRIF®, 1 case of grade 4 ALT with gefitinib. ALT=alanine15 aminotransferase; AST=aspartate transaminase; ILD=interstitial lung disease. Park K et al. Abstract LBA2 and oral presentation. European Society for Medical Oncology (ESMO) ASIA, Singapore, 20th December 2015. 1 (0.6)






