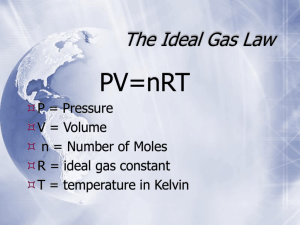
Combined Gas Laws and Dalton's Law of Partial
advertisement

Today… 1) Addressing Some CURIOUS questions 2) Take-up Homework 2) Minds-On-Making Connections to Charles Law and Gay Lussacs Law 3) Combined Gas law and Dalton’s Law of Partial Pressures Celsius and Kelvin Scales If you double the degrees Celsius, what happens to the temperature in Kelvin? • Graph of four experiments in which the same amount of gas was used and data were collected at four different pressures (P1 to P4). • The solid lines represent actual data, dashed lines represent extrapolated values. • All of the plots intersect at −273.15°C. Why Kelvin and Not Celsius for gas temperature? Identify the x and y-intercepts for each graph. (A) Using the Celsius temperature scale produces straight-line graphs that have three different y-intercepts. (not a direct proportion, doubling temp does not double volume) (B) Using the Kelvin temperature scale produces straight-line graphs with the same y-intercept (direct proportion, y-intercept 0, doubling temp doubles the volume) Combined Gas Laws and Dalton’s Law of Partial Pressures The Combined and Ideal Gas Laws • All three variables can be changed at the same time: Temperature, Pressure, and Volume. • A combination of Boyle’s and Charles’s law. • When the mass (amount) of gas is constant you can use: P V PV 1 T1 1 = 2 T2 2 Different forms of Equations P1V1 = P2V2 T1 T2 V 1 = P 2V 2 T 1 P1T2 T 1 = P 1V 1 T 2 V2 P2 P 1 = P 2V 2 T 1 T2 V1 V 2 = P 1V 1 T 2 T 1 P2 T 2 = P 2V 2 T 1 V 1 P1 P2 = P1V1 T2 T1 V2 pressure and volume of a given amount of gas are inversely proportional to each other, and directly proportional to the Kelvin temperature of the gas. The Combined Gas law Sample Problem: • A weather balloon with a volume of 55.0 L is filled with hydrogen gas at a pressure of 98.5 kPa and a temperature of 13ºC. When the balloon is released, it rises to the stratosphere, where the temperature is -48ºC and the pressure is 19.7 kPa. What is the volume of the balloon under these conditions? Plan your strategy Act on your strategy -None of them remain constant! -Which law: Combined Gas Law P1V1/T1 = P2V2/T2 -Isolate the variable you are looking for (in this case it is V2) -Remember to convert the temperatures into Kelvin. V2 = P1V1T2/P2T1 Plug the numbers (and units) of the known variables into the equation. V2 = (98.5 kPa)(55.0 L)(225 K)/(286 K)(19.7 kPa) = 216 L Conclusion: The volume at -48ºC and 19.7 kPa is 216 L. Class work Worksheet: Combining Gas Laws Dalton’s Law of Partial Pressures Gas Mixtures: In a mixture of gases, each gas exerts a pressure. The pressure exerted by a single gas in a mixture is called the Partial pressure of the gas. Dalton observed…. Law of Partial Pressures: The total pressure of a Mixture of gases is equal to the sum of the partial pressures of the component gases (if the gases do not react chemically). Mathematically this means that if a mixture is made of two gases, A and B, then: Pt = PA + PB Pt = Total pressure PA = Partial pressure of gas A PB = Partial pressure of gas B • When two separate gases originally at the same temperature are mixed, the temperature—and thus the average speed of the gases—does not change. Only the total number of molecules in the container increases. • Therefore there are more molecules colliding with the walls of the container and, thus, the pressure is higher. Pressure of a Gas • The pressure of a gas in a mixture is also related to the amount of molecules of that gas in the mixture. Mathematically this can be stated as: P = P x n A A nA n total total n total is called the mole fraction of gas A. Mole Fraction of a Gas the number of moles of gas A divided by the total number of moles of ALL gases in the mixture. Recap of Key Formulas PT = PA + PB PT = Total pressure PA = Partial pressure of A PB = Partial pressure of B _nA_ = mole fraction A ntotal nA = moles of gas A ntotal = Total moles of gas PA = Pt x nA ntotal PA = partial pressure of A Pt = total pressure nA = moles of gas A ntotal = total moles of gas Partial Pressure Sample Problem A mixture of 6.0 g of argon gas and 8.0 g of oxygen gas has a total pressure of 66 kPa. Calculate the partial pressure exerted by each gas. Plan your strategy Act on your strategy -This is a partial pressure problem PT = PAr + PO2 PAr = Ptotal x nAr/ntotal PO2 = Ptotal x nO2/ntotal i) -Convert each mass into moles and find ntotal. i) nAr = 6 g/39.95 g/mol = 0.15 mol nO2 = 8 g/32 g/mol = 0.25 mol ntotal = 0.25 mol + 0.15 mol = 0.40 mol ii) -Find the mole fraction of each gas. nA = mole fraction A ntotal -To find the partial pressure of each gas: PA = Pt x nA ntotal ii) nAr = mole fraction Ar = 0.15 mol/0.40 mol = 0.375 ntotal nO2 = mole fraction O2 = 0.25 mol/0.40 mol = 0.625 ntotal PAr = Pt x nAr = 66 kPa x 0.375 = 25 kPa ntotal PO2 = Pt x nO2 = 66 kPa x 0.625 = 41 kPa ntotal Conclusion: To check, add both partial pressures together. If their sum is equal to your total pressure (i.e. 66 kPa), then you have done the problem correctly and successfully!