OSMOSIS and DIFFUSION
advertisement
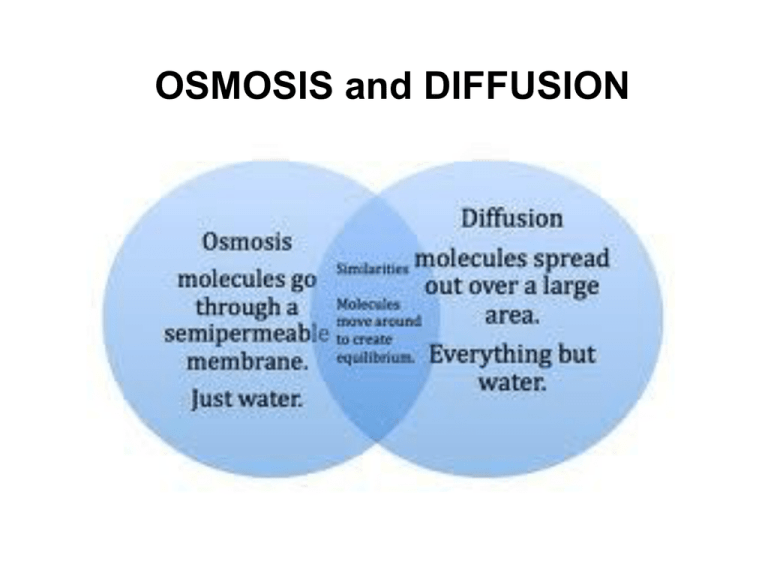
OSMOSIS and DIFFUSION Solute – Solvent - Solution Solute – Solvent - Solution Solute – Solvent - Solution Solute – Solvent - Solution Molecules are always moving Molecules move randomly and bump into each other and other barriers Concentration gradient Concentration Gradient - change in the concentration of a substance from one area to another. Diffusion Molecules in solution tend to slowly spread apart over time. This is diffusion. T1 T2 T3 Diffusion • • Movement of molecules from an area of high concentration to an area of lower concentration. Factors that affect the rate of diffusion: size of molecules, size of pores in membrane, temperature, pressure, and concentration. Diffusion concentrated, high energy molecules [High] [Low] diffuse, low energy molecules Diffusion will continue until equilibrium is reached. This means there will be an equal distribution of molecules throughout the space. This is why food coloring moves throughout a beaker of water; why odors smell strong at first and then disappear over time. Equilibrium, a result of diffusion, shows the uniform distribution of molecules of different substances over time as indicated in the above diagram. Which molecules will diffuse in each of the figures below? 1 2 5 3 6 4 ANSWERS 1 2 3 4 No Movement 5 6 No Movement Osmosis • Osmosis is the movement of WATER across a semi-permeable membrane • At first the concentration of solute is very high on the left. • But over time, the water moves across the semipermeable membrane and dilutes the particles. Osmosis – A Special kind of Diffusion Diffusion of water across a semi-permeable membrane (a barrier that allows some substances to pass but not others). The cell membrane is such a barrier. Small molecules pass through – ex: water Large molecules can’t pass through – ex: proteins and complex carbohydrates Hypotonic – The solution on one side of a membrane where the solute concentration is less than on the other side. Hypotonic Solutions contain a low concentration of solute relative to another solution. Hypertonic – The solution on one side of a membrane where the solute concentration is greater than on the other side. Hypertonic Solutions contain a high concentration of solute relative to another solution. Over time molecules will move across the membrane until the concentration of solutes is equal on both sides. This type of solution is called ISOTONIC. • Cytoplasm is a solution of water and solids (solutes dissolved in the water). • Water moves into and out of cells because of the different concentrations of the solutes. • Different kinds of cells react differently depending on the solution they are in. • Below are examples of red blood cells in different types of solutions and shows what happened to the red blood cells. PLANT CELLS Hypotonic Solution Turgor Pressure builds in the cell and causes osmosis to stop because of the rigid cell wall. Hypertonic Solution Plants will wilt when cells lose water through osmosis. ELODEA CELLS As viewed under the microscope PASSIVE TRANSPORT Passive transport occurs without expenditure of energy. Molecules move using their own kinetic energy . Diffusion and osmosis are examples of passive transport. Passive transport allows cells to get water, oxygen and other small molecules that they need. It also allows the cell to get rid of waste such as carbon dioxide. DIFFUSION OSMOSIS THE END BELLRINGER Dec. 8th Review Questions Write Questions AND Answers. Page 104 #7 and 10 QUAN both Page 106 #4 ANSWERS #7 For each pair of terms, explain how the meanings of the terms differ. Cellular Respiration and Fermentation According to our textbook on pages 95 through 97, cellular respiration differs from fermentation because cellular respiration is the process by which cells use oxygen to produce energy from food and fermentation breakdowns food without the use of oxygen. ANSWERS #10 Before the energy in food can be used by a cell, the energy must first be transferred to molecules of a. Proteins b. Carbohydrates c. DNA d. ATP ANSWERS #4 EGG & VINEGAR OSMOSIS You will need to designate someone to… • Measure the circumference of the egg BEFORE and AFTER the experiment. • To measure the needed volume of vinegar (200 mL) • To report the data from the group with to the class. • To collect and hand-in ALL COMPLETED lab reports to the teacher MAKING OBSERVATIONS • List 10 observations about the object below in your notebook. You will need this for your next lab. Ticket-into-Lab.