Sodium Carbonate Performance Assessment
advertisement

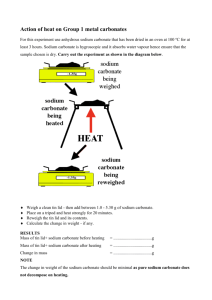
How Much Baking Soda Do I Need? Performance Assessment Stoichiometry How much NaHCO3 do you need? Background information: Due to the widespread use of sodium bicarbonate (commonly called baking soda) in many food products, the thermal decomposition reaction has been studied extensively by food chemists. Baking soda is used to prepare cakes in order to insure that cakes “rise” as they bake. As the temperature of the cake batter reaches approximately 50oC, the baking soda decomposes and carbon dioxide is released. The use of baking soda is especially popular in pancakes and waffles since the high cooking temperatures of 350-450oF (175-230oC) cause the carbon dioxide to be liberated before the dough has set. Thus, the batter rises before it sets, and we get a light and tasty finished product. NaHCO3, baking soda, breaks down to form sodium carbonate (solid), water (liquid), and carbon dioxide (gas). The unbalanced reaction is written below. NaHCO3 (s) Na2CO3 (s) + H2O (g) + CO2 (g) Problem: The goal of the lab is to figure out how much baking soda you will need to start with in order to produce a target amount of sodium carbonate. The target amount of sodium carbonate produced will be given to you by the teacher. How much NaHCO3 do you need? Background information: Due to the widespread use of sodium bicarbonate (commonly called baking soda) in many food products, the thermal decomposition reaction has been studied extensively by food chemists. Baking soda is used to prepare cakes in order to insure that cakes “rise” as they bake. As the temperature of the cake batter reaches approximately 50oC, the baking soda decomposes and carbon dioxide is released. The use of baking soda is especially popular in pancakes and waffles since the high cooking temperatures of 350-450oF (175-230oC) cause the carbon dioxide to be liberated before the dough has set. Thus, the batter rises before it sets, and we get a light and tasty finished product. NaHCO3, baking soda, breaks down to form sodium carbonate (solid), water (liquid), and carbon dioxide (gas). The word equation is written below. sodium bicarbonate sodium carbonate + water + carbon dioxide Problem: The goal of the lab is to figure out how much baking soda you will need to start with in order to produce a target amount of sodium carbonate. The target amount of sodium carbonate produced will be given to you by the teacher. Procedure: • Balance equation for the reaction of the decomposition of baking soda. I need to produce ______ grams of Na2CO3(s). • Calculate the amount of NaHCO3 you will need to start with in order to produce the target amount. Show your work and record the amount in your data section. • Set up a ring stand with an iron ring and wire gauze. Place the Bunsen burner on the stand. • Take the mass of the empty evaporating dish. ___________g. Tare/zero the scale. • Measure the amount of baking soda you calculated into the evaporating dish and place on top of the wire gauze for heating. • Heat the mixture for 3-5 minutes, but no more than that. Then let the evaporating dish cool. ~2 minutes • Bring evaporating dish to teacher to weigh and determine your accuracy. Data: Target amount of Na2CO3 produced _______________ grams. (theoretical yield) Actual amount of Na2CO3 produced _______________ grams. (actual yield) Amount of NaHCO3 calculated __________________ grams. Sodium bicarbonate decomposes upon heating to yield solid sodium carbonate, water and carbon dioxide gases Performance Assessment: Determine how much baking soda you must decompose to produce a specified amount of sodium carbonate. You will only get ONE attempt. You will be able to work with one partner to complete this task. I will assign your group an amount of sodium carbonate to produce. You must balance the equation, show your calculations, record mass of empty crucible. I will weigh the final product and give your grade based on how closely you are to the target given. 1 point deduction for every 5% off of the target. This is worth 10 points. Sodium bicarbonate decomposes upon heating to yield solid sodium carbonate, water and carbon dioxide gases Performance Assessment: Determine how much baking soda you must decompose to produce a specified amount of sodium carbonate. You will only get ONE attempt. You will be able to work with one partner to complete this task. I will assign your group an amount of sodium carbonate to produce. You must balance the equation, show your calculations, record mass of empty crucible. I will weigh the final product and give your grade based on how closely you are to the target given. 1 point deduction for every 5% off of the target. This is worth 10 points. Names______________________________________________ Names______________________________________________ A) Mass of sodium carbonate to produce A) Mass of sodium carbonate to produce 1.50 g 2.00 g B) Final mass of crucible + sodium carbonate ____________ B) Final mass of crucible + sodium carbonate ____________ C) Mass of sodium bicarbonate to start with C) Mass of sodium bicarbonate to start with D) Mass of empty crucible ____________ _________________ E) Calculation of mass of sodium bicarbonate needed: (using proper set-up + units) D) Mass of empty crucible ____________ _________________ E) Calculation of mass of sodium bicarbonate needed: (using proper set-up + units) _________________ _________________ F) Actual value of sodium carbonate produced ___________ F) Actual value of sodium carbonate produced ___________ G) Percent Error (show calculation) G) Percent Error (show calculation) _________________ _________________ Names______________________________________________ Names______________________________________________ A) Mass of sodium carbonate to produce A) Mass of sodium carbonate to produce 2.50 g 3.00 g B) Final mass of crucible + sodium carbonate ____________ B) Final mass of crucible + sodium carbonate ____________ C) Mass of sodium bicarbonate to start with C) Mass of sodium bicarbonate to start with D) Mass of empty crucible ____________ _________________ E) Calculation of mass of sodium bicarbonate needed: (using proper set-up + units) D) Mass of empty crucible ____________ _________________ E) Calculation of mass of sodium bicarbonate needed: (using proper set-up + units) _________________ _________________ F) Actual value of sodium carbonate produced ___________ F) Actual value of sodium carbonate produced ___________ G) Percent Error (show calculation) G) Percent Error (show calculation) _________________ _________________ Names______________________________________________ Names______________________________________________ A) Mass of sodium carbonate to produce A) Mass of sodium carbonate to produce 3.50 g 4.00 g B) Final mass of crucible + sodium carbonate ____________ B) Final mass of crucible + sodium carbonate ____________ C) Mass of sodium bicarbonate to start with C) Mass of sodium bicarbonate to start with D) Mass of empty crucible ____________ _________________ E) Calculation of mass of sodium bicarbonate needed: (using proper set-up + units) D) Mass of empty crucible ____________ _________________ E) Calculation of mass of sodium bicarbonate needed: (using proper set-up + units) _________________ _________________ F) Actual value of sodium carbonate produced ___________ F) Actual value of sodium carbonate produced ___________ G) Percent Error (show calculation) G) Percent Error (show calculation) _________________ _________________ Names______________________________________________ Names______________________________________________ A) Mass of sodium carbonate to produce A) Mass of sodium carbonate to produce 4.50 g 5.00 g B) Final mass of crucible + sodium carbonate ____________ B) Final mass of crucible + sodium carbonate ____________ C) Mass of sodium bicarbonate to start with C) Mass of sodium bicarbonate to start with D) Mass of empty crucible ____________ _________________ E) Calculation of mass of sodium bicarbonate needed: (using proper set-up + units) D) Mass of empty crucible ____________ _________________ E) Calculation of mass of sodium bicarbonate needed: (using proper set-up + units) _________________ _________________ F) Actual value of sodium carbonate produced ___________ F) Actual value of sodium carbonate produced ___________ G) Percent Error (show calculation) G) Percent Error (show calculation) _________________ _________________ Names______________________________________________ Names______________________________________________ A) Mass of sodium carbonate to produce A) Mass of sodium carbonate to produce 1.75 g 2.75 g B) Final mass of crucible + sodium carbonate ____________ B) Final mass of crucible + sodium carbonate ____________ C) Mass of sodium bicarbonate to start with C) Mass of sodium bicarbonate to start with D) Mass of empty crucible ____________ _________________ E) Calculation of mass of sodium bicarbonate needed: (using proper set-up + units) D) Mass of empty crucible ____________ _________________ E) Calculation of mass of sodium bicarbonate needed: (using proper set-up + units) _________________ _________________ F) Actual value of sodium carbonate produced ___________ F) Actual value of sodium carbonate produced ___________ G) Percent Error (show calculation) G) Percent Error (show calculation) _________________ _________________ Names______________________________________________ Names______________________________________________ A) Mass of sodium carbonate to produce A) Mass of sodium carbonate to produce 2.75 g 3.25 g B) Final mass of crucible + sodium carbonate ____________ B) Final mass of crucible + sodium carbonate ____________ C) Mass of sodium bicarbonate to start with C) Mass of sodium bicarbonate to start with D) Mass of empty crucible ____________ _________________ E) Calculation of mass of sodium bicarbonate needed: (using proper set-up + units) D) Mass of empty crucible ____________ _________________ E) Calculation of mass of sodium bicarbonate needed: (using proper set-up + units) _________________ _________________ F) Actual value of sodium carbonate produced ___________ F) Actual value of sodium carbonate produced ___________ G) Percent Error (show calculation) G) Percent Error (show calculation) _________________ _________________ Names______________________________________________ Names______________________________________________ A) Mass of sodium carbonate to produce A) Mass of sodium carbonate to produce 4.25 g 4.75 g B) Final mass of crucible + sodium carbonate ____________ B) Final mass of crucible + sodium carbonate ____________ C) Mass of sodium bicarbonate to start with C) Mass of sodium bicarbonate to start with D) Mass of empty crucible ____________ _________________ E) Calculation of mass of sodium bicarbonate needed: (using proper set-up + units) D) Mass of empty crucible ____________ _________________ E) Calculation of mass of sodium bicarbonate needed: (using proper set-up + units) _________________ _________________ F) Actual value of sodium carbonate produced ___________ F) Actual value of sodium carbonate produced ___________ G) Percent Error (show calculation) G) Percent Error (show calculation) _________________ _________________ Names KEY A) Mass of sodium carbonate to produce Names______________________________________________ 4.25 g A) Mass of sodium carbonate to produce 4.75 g B) Final mass of crucible + sodium carbonate ____________ B) Final mass of crucible + sodium carbonate ____________ C) Mass of sodium bicarbonate to start with ____________ C) Mass of sodium bicarbonate to start with D) Mass of empty crucible _________________ D) Mass of empty crucible E) Calculation of mass of sodium bicarbonate needed: (using proper set-up + units) 2 NaHCO3(s) xg Na2CO3(s) 4.25 g x g NaHCO3 = 4.25 g Na2CO3 ____________ + H2O(g) 1 𝑚𝑜𝑙 𝑁𝑎2𝐶𝑂3 106 𝑔 𝑁𝑎2𝐶𝑂3 + _________________ E) Calculation of mass of sodium bicarbonate needed: (using proper set-up + units) CO2(g) 2 𝑚𝑜𝑙 𝑁𝑎𝐻𝐶𝑂3 1 𝑚𝑜𝑙 𝑁𝑎2𝐶𝑂3 84 𝑔 𝑁𝑎𝐻𝐶𝑂3 1 𝑚𝑜𝑙 𝑁𝑎𝐻𝐶𝑂3 6.74 g Na2CO3 _________________ _________________ F) Actual value of sodium carbonate produced ___________ F) Actual value of sodium carbonate produced ___________ G) Percent Error (show calculation) G) Percent Error (show calculation) _________________ Multiply mass (1.5849) _________________