Interfacial Forces in Active Nanodevices
advertisement

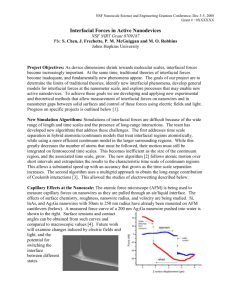
Interfacial Forces in Active Nanodevices (NIRT 0709187) S. Chen, S. Cheng, J. Frechette, R. Gupta, J. Liu, J. Ma, P. M. McGuiggan, M. O. Robbins: Johns Hopkins University Project Overview As device dimensions shrink into the nanometer range, interfacial forces become increasingly important. At the same time, traditional continuum theories of interfacial forces become inadequate, and fundamentally new phenomena appear. The goals of our project are to determine the limits of traditional theories, identify new interfacial phenomena, develop general models for interfacial forces at the nanometer scale, and explore processes that may enable new active nanodevices. To achieve these goals we are developing and applying new experimental and theoretical methods that allow measurement of interfacial forces on nanowires and in nanometer gaps between solid surfaces and the control of these forces using electric fields and light. Algorithmic Development: Multi-Timescales Algorithmic Development: Multi-Grid Coulomb Method A continuum-atomistic multi-timescale algorithm was developed [1]. Interfacial regions are treated atomistically, while a continuum description is used for bulk regions. The two are coupled through an overlap region. There remains a large gap between the time scales of atomic and bulk regions. The new algorithm integrates atomistic equations for short intervals and then extrapolates over the bulk time interval. Studies of electrowetting require efficient algorithms for the long-range interaction between charges. An efficient multi-grid method has been developed. It has enabled the studies of electrowetting described below, as well as simulations of electro-osmosis. Figures below show the charge distribution and effect on flow rate Q in rough channels. Effect of roughness on flow rate Charge density distribution Tests of Couette flow driven by an oscillating wall show that substantial speedups can be achieved and that errors decrease as the separation between time scales grows. 1. J. Liu, S. Chen. X. Nie & M. O. Robbins, Commun. Comput. Phys. 4, 1279 (2008). Wetting Measurements of Nanowires by AFM Simulations of Nanocapillaries: Electrowetting at Nanoscales: • Generic behavior studied first with Lennard-Jones interactions Liquid – short chain molecules with FENE bonds LJ units: energy e ~ 0.01eV, length s ~ 0.3nm, force e /s ~ 5pN. • Study effect of atomic structure of surfaces Rigid spheres (8 ~ 120nm), bent or cut, crystalline or amorphous Rigid or elastic substrate, (111) surface of fcc • Control q through solid-liquid interactions • Compare adhesive force and internal capillary pressure to continuum theory. • Relate differences to molecular scale properties and structure. 50 nm radius InAs nanowire2 250 nm radius Si 200 nm radius Ag2Ga nanowire4 nanowire3 Measuring the entire force curve as a nanowire is pushed/pulled though an air/fluid interface gives independent information about interfacial tension, contact angle, dynamic contact angles and hysteresis[5]. Future work will examine changes induced by electric fields and light, and the potential for switching the interface between different states. Capillary force on rods Can ignore gravity for small rods R ≡ r (rg/g)0.5 < 1 Away from end: F/r = 2p g cosq Contact angle hysteresis → Measure different angles as advance qadv and recede qrec Both vary with rate of motion Interface pinned at end. Peak force Fmax = 2pgr Measured force on 200nm Ag2Ga nanowire pushed into and retracted from water interface Forces consistent with bulk surface tension and contact angles: θadv = 58°, θrec = 47° r z θ Φ = 90 - θ 4 z r sin ln g E (1 cos R Water detaches z r EWOD with 12.68 m PDMS on Gold Results for capillary force F on sphere: 40 • At large h, exact theoretical results and the commonly used circle approximation are almost identical. Both are consistent with MD results. • At h < 12σ ~ 4nm, MD results deviate from continuum. There are large oscillatory forces related to layering of fluid molecules that vary with R. • The contributions to F can be resolved spatially into components from the surface tension at the edge of the drop, the Laplace pressure at intermediate r, and structural forces in a central layered region. • Discrepancies from continuum theory remain even after removing the oscillatory component. • Disjoining pressure effects lead to non-hydrostatic pressures in the outer region of the drop. • The pressure in the plane of the drop is consistent with bulk expressions for Laplace pressure and the bulk g. The adhesive force is determined by the z-component of the pressure, which is systematically less negative. Young-Lippmann Equation Fit 60 0.4 ΔV Fringes of Equal Chromatic Order (FECO) Water contact Transmitted spectrograph light with camera objective 70 0.2 80 saturation 0 90 100 -0.2 0 50 100 150 200 250 Force with layering term removed The surface force apparatus (SFA) allows study of liquids between surfaces with nanometer separation. The thickness can be measured optically with subnanometer resolution. Simulations of Electrowetting at Nanoscales The new multigrid Coulomb method described above allowed tests of the Young-Lippmann equation in droplets as small as 15s (~5nm) in radius. Short chain molecules like those in capillary adhesion simulations were used. The density contours below show the decrease in contact angle as the number of charges and the associated voltage increase. The charge remains highly localized at the surface of the insulator. The Young-Lippmann equation describes the changes in q in these nanoscale drops. As in macroscopic experiments, there is a saturation at large voltages. Increasing the chain length increases the saturation voltage by preventing molecules from evaporating under the high electrostatic force. This is a new mechanism for saturation Young-Lippmann Droplet density contours Current work shows that films can be condensed and evaporated by an applied field, creating another mechanism for controlling fluid geometry at nanometer scales. Piezo retract disks spring White light Image analysis Double cantilever spring (stiff) Helical spring (soft) 5. McGuiggan PM, Wallace JS (2006) J. Adhesion, 82: 997-1011. Lyons CJ, Elbing E, Wilson IR (1984) J. Coll. Int’ Sci., 102: 292-294. R v r1 θ l xL 110 300 Voltage (V) SFA experiments can measure the capillary forces described above and changes in force from electrowetting. Applying voltages to patterned electrodes on the mica surfaces can also change the droplet configuration via electrowetting effects. approach 50 Experimental Data 0.6 Nanoscale Electrowetting in the Surface Force Apparatus Surface Forces Apparatus Applying a voltage V between a fluid and an electrode covered by an insulator of dielectric ed and thickness d leads to a new contact angle qEW At macroscopic scales this is described by the Young-Lippmann equation: cos qEW = cos q0 + e0ed V2/2dg , where q0 is the equilibrium angle and g the liquid surface tension. The curves below show mm scale measurements by our team Contact Angle, q 2. Prepared by Brian Swartzentruber, Doug Pete, and Tom Picraux as part of a Sandia CINT User Proposal #U2008A160: Fabrication of Nanowires attached to AFM cantilevers 3. Prepared by Frank Zhu at Johns Hopkins University using a FIB to mill the nanowire from a Si cantilever 4. Prepared from solution by NaugaNeedles, LLC Active nanodevices require means of changing capillary forces. We are exploring control of q by electric fields and optical illumination. R cos q The atomic force microscope (AFM) is being used to measure capillary forces on nanowires as they are pulled through an air/liquid interface. The effects of surface chemistry, nanowire roughness and radius, and velocity are being studied. Examples of nanowires under study are shown below. Charge density contours