Feb 18 SI
advertisement

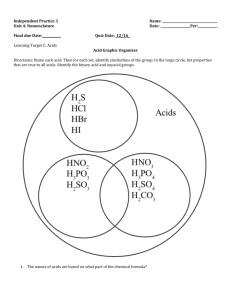
SI: Chem 178 Ch 16 Leader: Emily Feb 18th, 2013 Concepts: In poly-protic acids (ex: H2SO4) is the second H EASIER or HARDER to remove than the first? Which of the above acids is strongest? Write out a Ka equation for one of the above acids. List two of the acids and write out their conjugate bases. How are Ka and Kb related in an acid/base pair? What is the generalized form of Kb? Problems: I have a solution of Hypochlorous acid at equilibrium. If its pH is 3.77, what are the concentrations of its components? Do you need an ICE table for this? If I started with 1.13M HClO, what would my % dissociation be? What is the pH of a solution of Benzoic Acid that had an initial HC7H5O2 concentration of 0.33? (Do you need an ICE table for this? Do you need the quadratic equation? Use the above table to find the Ka.) What is it’s % ionization? What is the pH of a 0.87M solution of NH3 with a Kb of 1.8x10^-5 ? New Stuff: As one moves down the periodic table from F to I, does the HX bond get weaker or stronger? Does this make a weaker or stronger acid? In the series of acids going from hypochlorous to perchloric acid, why are is perchloric the strongest? (See structures on board, consider charge and resonance…)What are these called?