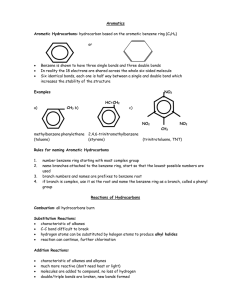
Benzene

20.8 Aromatic Hydrocarbons
- 6 carbon ring w/double bonds
-”benzene ring”
- smell nice (cinnamon and vanilla)
-different than cyclo- hydrocarbons
Cyclohexane
Puckered
109.5
o <‘s
More H
Saturated
Benzene
Flat
120 o <‘s
Fewer H
Unsaturated
Figure 20.8: Two Lewis structures for the benzene ring .
Figure 20.8: Shorthand notation for benzene rings.
Figure 20.9: The bonding in the benzene ring is a combination of different Lewis structures.
Figure 20.10:
Names of some common monosubstituted benzenes.
20.10 Functional Groups
Hydrocarbon derivatives = primarily
HC’s but have additional atoms or groups of atoms called functional groups. A functional group is a reactive portion of a molecule that undergoes predictable reactions.
Common functional groups
alcohols
ethers
aldehydes
ketones
carboxylic acids
esters
alcohol
hydroxyl group (-OH)
Some examples are
OH
CH
3
CH CH
3
2-propanol
CH
3
OH methanol
CH
3
CH
2
OH ethanol
Methanol = gasoline
Ethanol = alcohol beverage
Isopropyl = rubbing alcohol
ether
oxygen “bridge”
An example is
CH
3
CH
2
O CH
2 diethyl ether
CH
3
(This is the most common ether, often called simply ether, used as an anesthetic.)
carboxylic acid
carboxyl group, -COOH
An example is O
CH
3
C OH ethanoic acid
ester
compound formed from a carboxylic acid, RCOOH, and an alcohol, R’OH.
The general structure is
O
R C O R'
aldehyde
carbonyl group (C=O) with at least one H atom.
An example is O
CH
3
CH ethanal
ketone
carbonyl group with two hydrocarbon
An example is
O
CH
3
CH
2
C
2-butanone
CH
3