Aromatics Aromatic Hydrocarbons H
advertisement
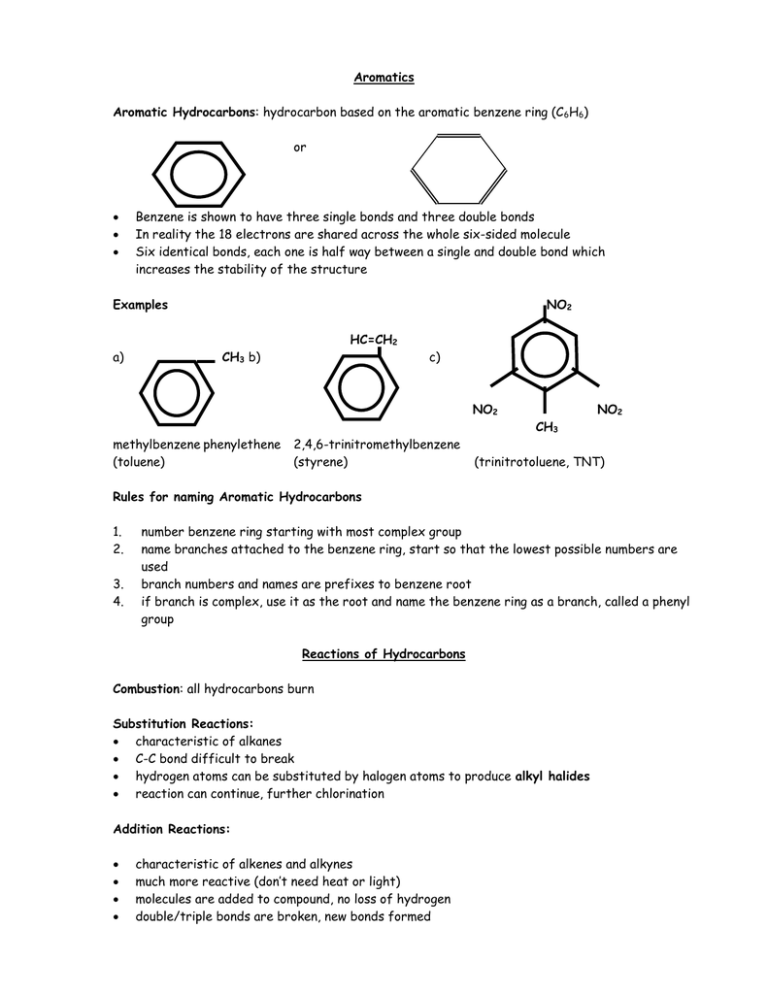
Aromatics Aromatic Hydrocarbons: hydrocarbon based on the aromatic benzene ring (C 6H6) or Benzene is shown to have three single bonds and three double bonds In reality the 18 electrons are shared across the whole six-sided molecule Six identical bonds, each one is half way between a single and double bond which increases the stability of the structure Examples NO2 HC=CH2 a) CH3 b) c) NO2 NO2 CH3 methylbenzene phenylethene (toluene) 2,4,6-trinitromethylbenzene (styrene) (trinitrotoluene, TNT) Rules for naming Aromatic Hydrocarbons 1. 2. 3. 4. number benzene ring starting with most complex group name branches attached to the benzene ring, start so that the lowest possible numbers are used branch numbers and names are prefixes to benzene root if branch is complex, use it as the root and name the benzene ring as a branch, called a phenyl group Reactions of Hydrocarbons Combustion: all hydrocarbons burn Substitution Reactions: characteristic of alkanes C-C bond difficult to break hydrogen atoms can be substituted by halogen atoms to produce alkyl halides reaction can continue, further chlorination Addition Reactions: characteristic of alkenes and alkynes much more reactive (don’t need heat or light) molecules are added to compound, no loss of hydrogen double/triple bonds are broken, new bonds formed Reactions Halogenation (Br2, Cl2) CH3 – CH = CH2 + Hydrogenation (H2) CH3 – CH = CH2 + Hydrohalogenation (hydrogen halides) CH3 – CH = CH2 + Hydration (H2O) CH3 – CH = CH2 + Markovnikov’s Rule: When two different atoms are added, the hydrogen bonds with the carbon that is already bonded to the most hydrogen atoms.








