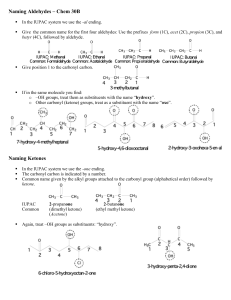
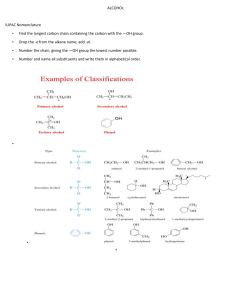
MLSINOR01, BSMT 1 - Lymphocytes Mr. Clint Eleazer Ricarse Short Background: Properties A l d e h y d e s are c o m p o u n d s w i t h a terminal carbonyl (H C = O ) m o i e t y, a n d a r e either u n s a t u r a t e d , c o n t a i n s o n e o r m o r e c a r b o n -c a r b o n d o u b l e b o n d s , o r s a t u r a t e d t h a t c o n t a i n s a single b o n d . O n e of the oldest k n o w n aldehydes wa s first produced in 1774 by Carl Wilhelm Scheele, although its structure wa s not completely completely understood until 60 years later wh e n Justus von Liebig determined its constitution, described its preparat i on f r o m e t h a n o l a n d g a v e t he n a m e “aldehydes” to the chemical group It is m ainly derived f r o m the dehydration of a l c o h o l s a n d is actually c o n s i d e r e d t h e m o s t important functional g ro u p . T h e reactivity of these c o m p o u n d s arises largely t h r o u g h t w o features of their structures: the polarity of the carbonyl group and the acidity of any ahydrogens that are present. Aldehydes are polar molecules and m a n y reagents s eek a t o m s wi t h a deficiency of electrons. The general formula for aldehydes is CnH2n+1 C H O or C n H 2 n O . Functional Groups One of the most important functional group in organic chemistry is the carbonyl group: The carbonyl group is present in the aldehydes and ketones. The carbon of the carbonyl group must be connected to two other atoms or groups. The compound is called an aldehyde when it is connected to a hydrogen and an alkyl group or aromatic ring (or to two hydrogens). Proper IUPAC/Common Naming (Steps) 1 . Aldehydes take their n a m e f r o m their parent alkane chains. T h e -e is r e m o v e d f r o m the e n d a n d is replaced with -al. 2. T h e aldehyde functional group is given the #1 numbering location and this number is not included in the n a m e . 3 . For the c o m m o n n a m e of aldehydes start w i t h the c o m m o n p ar e n t c h a i n n a m e a n d a d d the suffix -aldehyde. Substituent positions are shown with Greek letters. 4 . W h e n the -C H O functional group is attached to a ring the suffix -car 5 . b a l d e h y d e is a d d e d , a n d t h e c a r b o n attached to that g r o u p is C 1 . The IUPAC system names are given o n top while the c o m m o n n a me is given o n the bottom in parentheses: There are s o me c o m m o n n a m e s that are still us ed and need to be memorized. R e c o g n i z i n g the patterns c a n b e helpful. T h e s e are the a l d e h y d e c o m m o n names to memorize. Writing of Structures A n aldehyde has a functional group of C H O . W h e n writing the structure, carbon (C ) is double b o n d i n g with o x y g e n (O ) a n d single b o n d i n g with hydrogen (H ). We should always k n o w that the c a r b o n in the functional g r o u p is a l w a y s the first carbon. The general formula of alkene is C n H 2 n + 1 and as aforementioned, the general formula for aldehyde will be CnH2n+1CHO or CnH2nO which will be a great basis in writing the structure. T h e structure w e see above is an example of a n aldehyde n a m e d as f or ma l de hyde w h i c h is the simplest aldehyde. We can see that the structure has a carbon a t o m w h i c h has single bonds to two hydrogen atoms in different sides a n d has a double b o n d with oxygen. In all other aldehydes, the carbonyl g r o u p is b o n d e d to o n e h y d r o g e n a n d o n e c a r b o n g r o u p . A carbonyl g r o u p is a f unctional g r o u p c o m p o s e d o f a c a r b o n a t o m d o u b l e -b o n d e d t o a n o x y g e n atom: C =O T h e structure w e see a b o v e is another e x a m p l e of a n a l d e h y d e k n o w n as acetaldehyde. W h e n writing its structure, the c a r b o n y l g r o u p (C = O ) is b o n d e d to o n e hydrogen and one carbon group. EXAMPLES: COMMON/IUPAC NAMES, STRUCTURES & USES Methanal ( IUPAC ) Formaldehyde (C o m m o n ): C H 2 O - It is naturally used as an aqueous solution containing 37% methanal, known as formalin. It contains a small a m o u n t of methanol a n d an inhibitor to prevent aldehyde f r o m forming long chain p o l y m e r s o n storage. It has o n e car b o n a t o m , t w o hydrogen atoms and one oxygen atom. Ethanal ( IU PA C ) Acetaldehyde (C o m m o n ):C H 3 C H O or C 2 H 4 O - used as starting mater ial a n d it h a s b e e n m a n u f a c t u r e d b y hydr ation of acetylene a n d oxidation o f e t h a n o l Benzenecarbaldehyde (I U PA C ) Benzaldehyde (c o m m o n ): C 6 H 5 C H O - A n organic c o m p o u n d consisting of a benzene ring with a formyl substituent. It is used in the production of d y e s , soaps a n d p e r f u m e s . It is also used in cakes a n d b a k e d g o o d s a s a l m o n d extract. Pentanal ( I U PA C ) Valeraldehyde (common): C5H10O -It is a s at u r at ed fatty a l d e h y d e c o m p o s e d from five carbon in a straight chain. It is used in flavorings, resin chemistry and rubber accelerators. Its smell is described as fermented, bready, fruity, nutty and berry. Butanal (IUPAC) butyraldehyde ( common): C H 3 (C H 2 )2 C H O - A p p e a r s as a clear liquid w i t h a p u n g e n t odor. Less dense that water an d insoluble in water, vapor heavier that air. It is also m a i n l y u s e d as a n intermediate in the production of synthetic resins, rubber vulcanization accelerators, solvents a n d plasticizers. Propanal ( I U PA C ) Propionaldehyde ( common ): C H 3 C H 2 C H O - It is the 3 carbons aldehyde. It is a colorless, flammable liquid with a slightly fruity odour. It is used in the manufacture of plastics, in the synthesis of rubber chemicals a n d a s a disinfectant a n d p r e s e r v a t i v e . 2- Chloropentanal (I U PA C ) achlorovaleraldehyd( c o m m o n ):C 5 H 9 C I O - S o m e h o w used to m a k e insulation and plastic d i n n e r w a r e . 3- Methylbutanal ( IUPAC ) isovaleraldehyde ( c o m m o n ): (C H3 )2 C H C H 2 C H O - It is butanal substituted by a methyl group at position 3 . It occurs as volatile constituent in olives. Also produced c o m m e r c i a l l y a n d is u s e d as a reagent for the p r o d u c t i o n o f p h a r m a c e u t i c a l s a n d pesticides. 4- Hydroxybutanal ( IUPAC ) Bhydroxybutyraldehyde ( c o m m o n ): C4H8O2 - T h e hydroxybutanal molecule contains a total of 1 3 b o n d s , 3 rotatable b o n d s , 1 d o u b l e b o n d (s), a a l d e h y d e (aliphatic ), 1 hydroxyl groups and 1 primary alcohols. It was formerly used in m e d i c i n e a s a h y p n o t i c a n d sedative. 2-Chlorobutanal (IUPAC ) B chlorobutyraldehyde ( c o m m o n ): C4H7CIO - A n alcohol based preservatives with no surfactant activity. It also elicits sedative- h y p n o t i c a n d w e a k local anesthetic actions. It is widely used as a c h e m i c a l preservative for injectable drugs, eye drops, m o u t h washes and cosmetics. Members: Exija, Lester John C. Managuit, Thrilla Dawn L. Paredes, May Catherine A. Pascual, Oriel D. Pillo, Aennylou Rojo, Aj Vhert Esmeralda, Myrel Joy A. Balitor, Glovin Adrian V. Genolos, Ronest Angel N. Guarino, Ana Mariel S. Isio, Carl John M. Magon, Angel Ann F. Navales, Henryl Jane A. General References: h t t p s : / / w w w. v e d a n t u . c o m / c h e m i s t r y / p h y s i c a l - p r o p e r t i e s - o f - a l d e h y d e s , h t t p s : / / w w w 2 . c h e m i s t r y. m s u . e d u / f a c u l t y / r e u s c h / v i r t t x t j m l / a l d k e t 1 . h t m h t t p s : / / s t u d y. c o m / a c a d e m y / l e s s o n / a l d e h y d e - d e f i n i t i o n - r e a c t i o n s - f o r m u l a - s t r u c t u r e . h t m l , h t t p s : / / b y j u s . c o m / c h e m i s t r y / a l d e h y d e s h t t p s : / / g u i d e s . h o s t o s . c u n y. e d u / c h e 1 2 0 / c h a p t e r 3