Chapter 20 Carboxylic Acids
advertisement

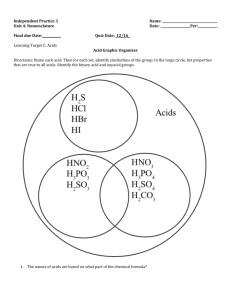
Chapter 12 Carboxylic Acids Introduction • Carbonyl (-C=O) and hydroxyl (-OH) on the same carbon is carboxyl group. • Carboxyl group is usually written -COOH. • Aliphatic acids have an alkyl group bonded to -COOH. • Aromatic acids have an aryl group. • Fatty acids are long-chain aliphatic acids. Chapter 20 2 Common Names • Many aliphatic acids have historical names. • Positions of substituents on the chain are labeled with Greek letters. Chapter 20 3 IUPAC Names • Remove -e from alkane (or alkene) name, add -oic acid. • The carbon of the carboxyl group is #1. Cl O Ph CH3CH2CHC OH H 2-chlorobutanoic acid H C C COOH trans-3-phenyl-2-propenoic acid (cinnamic acid) Chapter 20 4 Naming Cyclic Acids • Cycloalkanes bonded to -COOH are named as cycloalkanecarboxylic acids. • Aromatic acids are named as benzoic acids. COOH COOH CH(CH3)2 OH 2-isopropylcyclopentanecarboxylic acid o-hydroxybenzoic acid (salicylic acid) Chapter 20 5 Dicarboxylic Acids • Aliphatic diacids are usually called by their common names (to be memorized). • For IUPAC name, number the chain from the end closest to a substituent. • Two carboxyl groups on a benzene ring COOH indicate a phthalic acid. Br HOOCCH2CHCH2CH2COOH 3-bromohexanedioic acid -bromoadipic acid COOH 1,3-benzenedicarboxylic acid m-phthalic acid Chapter 20 6 Structure of Carboxyl • Carbon is sp2 hybridized. • Bond angles are close to 120. • O-H eclipsed with C=O, to get overlap of orbital with orbital of lone pair on oxygen. Chapter 20 7 Boiling Points Higher boiling points than similar alcohols, due to dimer formation. Acetic acid, b.p. 118C Chapter 20 8 Melting Points • Aliphatic acids with more than 8 carbons are solids at room temperature. • Double bonds (especially cis) lower the melting point. Note these 18-C acids: – Stearic acid (saturated): 72C – Oleic acid (one cis double bond): 16C – Linoleic acid (two cis double bonds): -5C Chapter 20 9 Solubility • Water solubility decreases with the length of the carbon chain. • Up to 4 carbons, acid is miscible in water. • More soluble in alcohol. • Also soluble in relatively nonpolar solvents like chloroform because it dissolves as a dimer. Chapter 20 10 Acidity Chapter 20 11 Resonance Stabilization Chapter 20 12 Substituent Effects on Acidity Chapter 20 13 Salts of Carboxylic Acids • Sodium hydroxide removes a proton to form the salt. • Adding a strong acid, like HCl, regenerates the carboxylic acid. O CH3 C OH NaOH O CH3 _ + C O Na HCl Chapter 20 14 Naming Acid Salts • Name the cation. • Then name the anion by replacing the -ic acid with -ate. Cl - CH3CH2CHCH2COO K + potassium 3-chloropentanoate potassium -chlorovalerate Chapter 20 15 Properties of Acid Salts • Usually solids with no odor. • Carboxylate salts of Na+, K+, Li+, and NH4+ are soluble in water. • Soap is the soluble sodium salt of a long chain fatty acid. • Salts can be formed by the reaction of an acid with NaHCO3, releasing CO2. Chapter 20 16 Purifying an Acid Chapter 20 17 Some Important Acids • Acetic acid is in vinegar and other foods, used industrially as solvent, catalyst, and reagent for synthesis. • Fatty acids from fats and oils. • Benzoic acid in drugs, preservatives. • Adipic acid used to make nylon 66. • Phthalic acid used to make polyesters. Chapter 20 18 Synthesis Review • Oxidation of primary alcohols and aldehydes with chromic acid. • Cleavage of an alkene with hot KMnO4 produces a carboxylic acid if there is a hydrogen on the double-bonded carbon. • Cleavage of an alkyne with ozone or hot permanganate. Chapter 20 19 Fischer Esterification • • • • Acid + alcohol yields ester + water. Acid catalyzed for weak nucleophile. All steps are reversible. Reaction reaches equilibrium. O COOH + H + CH3CH2OH Chapter 20 COCH2CH3 + HOH 20 Amides from Acids • Amine (base) removes a proton from the carboxylic acid to form a salt. • Heating the salt above 100C drives off steam and forms the amide. O O O C OH CH NH + 3 2 C O- +NH CH 3 3 C NHCH 3 heat Chapter 20 + H2O 21 Reduction to 1 Alcohols • Use strong reducing agent, LiAlH4. • Borane, BH3 in THF, reduces carboxylic acid to alcohol, but does not reduce ketone. Chapter 20 22 Reduction to Aldehyde • Difficult to stop reduction at aldehyde. • Use a more reactive form of the acid (an acid chloride) and a weaker reducing agent, lithium aluminum tri(t-butoxy)hydride. O O CCl LiAl[OC(CH3)3]3H C Chapter 20 H 23 Esters from Acid Chlorides • Acid chlorides react with alcohols to give esters in good yield. • Mechanism is nucleophilic addition of the alcohol to the carbonyl as chloride ion leaves, then deprotonation. O O CCl COCH3 + CH3OH + HCl Chapter 20 24 End of Chapter 12 Chapter 20 25