PPT - Ch 6.3b - Naming Covalent Compounds
advertisement

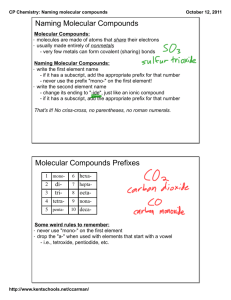
Bell Ringer Draw the Molecule & give the Formula for: – Carbon and 2 Sulfurs – Hydrogen, Oxygen, and Chlorine – Carbon, two Chlorines, 2 Fluorines – Phosphorus, Hydrogen, Sulfur Molecular Compounds: Formulas & Naming Ch 6.3b - Covalent Bonds Describing Molecular Compounds What information do the name and formula of a molecular compound provide? – Ionic Bonds (Ionic Compounds) - Describing Molecular Compounds What information do the name and formula of a molecular compound provide? – Ionic Bonds (Ionic Compounds) Name – Type of elements in the compound Formula – Ratio/Number of atoms of each element in the compound. Describing Molecular Compounds Covalent Bonds (Molecular Compounds) – Name & Formula - the type and number of atoms in a molecule of the compound. Naming Molecular Compounds Naming Order: Step 1: – Element farthest to the left named 1st. Most Metallic Elements in the same group more metallic element is closer to the bottom of the group. Name does not change! (just like in ionic) – Formulas are written same way – Example: Sulfur is named before chlorine SCl2 Naming Molecular Compounds Exception: Oxygen & Halogens – – Oxygen comes 2nd when combined with lower-electronegativity halogens (Group 17) Example: ClO2 (electronegativity: Cl = 3.0; O = 3.5) Naming Molecular Compounds Step 2: – Second element “ide” ending (like the anion in an ionic compound) Examples: – – Chlorine chloride Oxygen oxide Naming Molecular Compounds Step 3: – Add prefixes to elements names to specify the number of atoms of each element in the compound. mono - one di - two tri - three tetra - four penta - five hexa - six hepta - seven octa - eight nona - nine deca - ten Naming Molecular Compounds Prefixes: • mono - one • di - two • tri - three • tetra - four • penta - five Example: N2O Dinitrogen monoxide N4O5 Tetranitrogen pentoxide Naming Molecular Compounds Exception to Step 3– – Only one atom of the 1st element = NO PREFIX! Carbon monoxide CO2 Carbon dioxide SO10 Sulfur decaoxide CO Naming Molecular Compounds More Examples: – Dinitrogen pentachloride N2Cl5 – Dinitrogen pentoxide N2O5 – Pentaphosphorous hexachloride P5Cl6 – Sulfur decaoxide SO10 Writing Molecular Formulas Step 1: – Write the element symbols (in order) Step 2: – Prefixes values subscripts No prefix = 1 atom (no subscript) – Only applies to 1st element in the formula Writing Molecular Formulas Examples: – Diphosphorus Pentoxide P2O5 – Silcon Dioxide SiO2 – Carbon Tetrachloride CCl4 – Trineptunium Octoxide Np3O8 You Try Give the formula for: – Oxygen Difluoride – Phosphorus Tribromide – Trisilicon Tetranitride Give the Name – NO2 – N2O3 – SO3 for: You Try - Answers Give the formula for: – Oxygen Difluoride OF2 – Phosphorus Tribromide – Trisilicon Tetranitride Give the Name – NO2 – N2O3 – SO3 for: You Try - Answers Give the formula for: – Oxygen Difluoride OF2 – Phosphorus Tribromide PBr3 – Trisilicon Tetranitride Give the Name for: – NO2 – N2O3 – SO3 You Try - Answers Give the formula for: – Oxygen Difluoride OF2 – Phosphorus Tribromide PBr3 – Trisilicon Tetranitride Si3N4 Give the Name for: – NO2 – N2O3 – SO3 You Try - Answers Give the formula for: – Oxygen Difluoride OF2 – Phosphorus Tribromide PBr3 – Trisilicon Tetranitride Si3N4 Give the Name for: – NO2 Nitrogen Dioxide – N2O3 – SO3 You Try - Answers Give the formula for: – Oxygen Difluoride OF2 – Phosphorus Tribromide PBr3 – Trisilicon Tetranitride Si3N4 Give the Name for: – NO2 Nitrogen Dioxide – N2O3 Dinitrogen Trioxide – SO3 You Try - Answers Give the formula for: – Oxygen Difluoride OF2 – Phosphorus Tribromide PBr3 – Trisilicon Tetranitride Si3N4 Give the Name for: – NO2 Nitrogen Dioxide – N2O3 Dinitrogen Trioxide – SO3 Sulfur Trioxide