UV-VIS Spectroscopy
advertisement

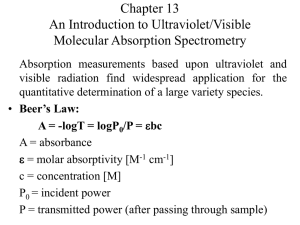
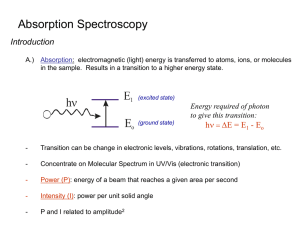
UV-VIS Spectroscopy Dr. AKM Shafiqul Islam Spectrometer Experiment Irradiance in Irradiance out Pathlength Irradiance, P, is measured in W·m-2 Transmittance, T, is the fraction of original light not P absorbed by the sample, or T 10kb P0 Where po = is power of incident light, p = power of transmitted light and b = path length • A light source can be a lamp, laser, or even a light bulb. A monochromator (“one color”) is used to select a particular wavelength. This is typically a grating, but can be a prism or filter. The light then passes through the sample, containing the analyte. Afterwards, the light is detected. Putting this in logarithmic form P log T log kb P0 In 1852, Beer and Bernard each stated that a similar law holds for the dependence of concentration, c P k c T 10 P0 Where k’ is a new constant P log T log k c P0 Combining these two laws which describes the dependence of T on both the path length and concentration P T 10 abc P0 Where a is a combined constant of k and k’ P log T log abc P0 It is more convenient to remove negative sign on right hand side, we get P0 1 A log T log log abc T by P The transmittance is given P %T 100 P0 or, T %T / 100 Now, we can write 100 A log log 100 log %T %T Absorbance and Beer’s Law Absorbance, A, the amount light absorbed by the sample is related to transmittance: P A log 0 log T P Beer’s law relates the absorbance of a chemical to its concentration: A bc b is the pathlength, typically in cm, and c is the concentration of the chemical species in M is the molar absorptivity, the unit that tells how much light is absorbed for a given wavelength. has units of M-1 cm-1 Wavelengths and Color Beer’s Law Assumptions The light being shined on the sample must be monochromatic (one color or wavelength) The analyte must not be participate in a concentration dependent equilibrium This isn’t a good technique for many weak acid systems, as dilution increases dissociation and HA and A- probably don’t have the same absorbance To do a Spectroscopic Analysis You need: A continuous light source A wavelength selector A sample cell A detector The sample cell is called cuvet and can be made of many substances Glass (good for visible) Quartz (UV-vis) NaCl/KBr (IR) Spectroscopic Procedure You may have a single-beam or double beam Single-beam instrument has one sample holder, you must swap blank and sample Double-beam instrument splits light output between two holders so you can measure blank and sample A baseline spectrum is a spectrum of a reference solution (solvent or reagent blank) We try to do an analysis at the λmax if we can Sensitivity is greatest at maximum absorbance Curve is relatively flat in case the monochromator drifts and is off by a little in wavelength The Single-Beam Spectrometer How Do UV spectrometers work? Rotates, to achieve scan Matched quartz cuvettes Sample in solution at ca. 10-5 M. System protects PM tube from stray light D2 lamp-UV Tungsten lamp-Vis Double Beam makes it a difference technique Two photomultiplier inputs, differential voltage drives amplifier. Optics of the Grating Monochromator The polychromatic light is separated into monochromatic wavelengths by diffraction. n = d(sin + sin ) Optics of the Grating Monochromator In the equation n = d(sin + sin ) n is the order of the diffraction n = 1, 2, 3 etc, d is the number of lines etched on the grating, is the angle of the incident beam and is the angle of the emerging beam. The photodiode array detector The photodiode array detector UV Instrumentation Key components: (1) Light Source (2) Monochromator (3) Sample/reference holder (4) Radiation detection (5) Readout device Spectroscopic Procedure We should always try to keep the absorbance reading of our sample below 1. Because % transmittance is related logarithmically with concentration, it means that from 1-99% transmittance we can detect ~ 2 orders of magnitude in analyte concentration. Any orders of magnitude greater than that will be detected in the range of 0-1% T. In order to maximize accuracy, you should dilute the solution if you have to so that the transmittance reading is not maxed out in that region. Spectroscopic Procedure You should always try to keep the absorbance reading of your sample below 1. Because % transmittance is related logarithmically with concentration, it means that from 1-99% transmittance you can detect ~ 2 orders of magnitude in analyte concentration. Any orders of magnitude greater than that will be detected in the range of 0-1% T. In order to maximize accuracy, you should dilute the solution if you have to so that the transmittance reading is not maxed out in that region. An Electronic Spectrum Make solution of concentration low enough that A≤ 1 (Ensures Linear Beer’s law behavior) 1.0 maxwith certain extinction UV Visible Even though a dual beam goes through a solvent blank, choose solvents that are UV transparent. Absorbance Can extract the value if conc. (M) and b (cm) are known UV bands are much broader than the photonic transition event. This is because vibration levels are superimposed on UV. 0.0 200 400 Wavelength, , generally in nanometers (nm) 800 Ultraviolet Spectroscopy 200-400 nm photons excite electrons from a bonding orbital to a * antibonding orbital. Conjugated dienes have MO’s that are closer in energy. A compound that has a longer chain of conjugated double bonds absorbs light at a longer wavelength. => Absorption Characteristics of Some Common Chromophores Chromophore Alkene Example Solvent Type of transition 177 13,000 * n-Heptane 178 196 225 10,000 2,000 160 * _ _ O n-Hexane 186 280 1,000 16 CH3CCH3 O ns* n* n-Hexane 180 293 Large 12 Ethanol 204 41 n* Water 214 60 n* Ethanol 339 5 n* n* C5H11C Carbonyl Amido max n-Heptane C6H13HC CH2 Alkyne Carboxyl max (nm) C CH3 CH3CH O CH3COH O ns* n* CH3CNH2 Azo H3CN NCH3 Nitro CH3NO2 Isooctane 280 22 Nitroso C4H9NO Ethyl ether 300 665 100 20 n* 270 12 n* Nitrate C2H5ONO2 Dioxane _ Solvents for UV (showing high energy cut-offs) Water 205 THF 220 CH3CN 210 CH2Cl2 235 C6H12 210 CHCl3 245 Ether 210 CCl4 265 EtOH 210 benzene 280 Hexane 210 Acetone 300 MeOH 210 Dioxane 220 Various buffers for HPLC, check before using. Deviation from Beer’s Law Beer’s law is only valid for low concentration, up to 10 mM; The intermolecular distances in a given solution will decrease, eventually reach a point at which neighboring molecules mutually affect the charge distribution of the other affect Chemical processes such as the reversible associationdissociation of analyte molecules, or the ionization of a weak acid in an unbuffered solvent. Instrumentation limitation-incident beam may be polychromatic . Background Correction -Processes other than analyte absorption result in significant decrease in the power of the incident beam; - Reference cell is used to correct these processes; - Reference cell is often prepared by adding distilled water to an absorption cell; - The reference cell is then placed in the path of the light beam, and the power of the radiation exiting the reference cell is measured and taken as P0 for the sample cell. Calibration Curves • Linear calibration curve; • Nonlinear calibration An Example--Pulegone Frequently plotted as log of molar extinction So at 240 nm, pulegone has a molar extinction of 7.24 x 103 Antilog of 3.86 O Example A solution containing a compound of formula weight 280 g/mol absorbed 65.0% of the light with 450 nm wavelength in a 2.00 cm cell at a concentration of 15.0 × 10-3 g/L. Calculate its molar absorptivity at 450 nm.