211 lec2
advertisement

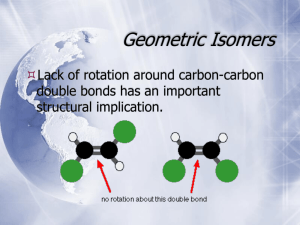
PHARMACEUTICAL ORGANIC CHEMISTRY LEC 2 QI: Arrange the following sets in order of decreasing priority -H, -C2H5, -CH3, -C(CH3)3, -CH(CH3)2 Problem 1: Lecture contents Geometrical Isomerism Optical Isomerism Polarimeter Chirality Chiral compounds Optical Isomerism Stereochemistry Optical isomerism An isomerism resulting from ability of certain molecules to rotate plane of polarized light -- the light is rotated either to the right or left right ( clockwise ) left ( anticlockwise ) + - d ( dexter ) dextro l ( laevous ) levo Polarimetry is a laboratory technique that measures the interaction between a compound and plane polarized light. Since enantiomers interact with plane polarized light differently, polarimetry can be used to distinquish between enantiomers. Optical Isomerism: Any material that rotates the plane of polarized light is said to be optically active. Optically active compound is nonsuperimposable on its mirror image. If a molecule is superimposable on its mirror image, the compound does not rotate the plane polarized light; it is optically inactive. Example: CH3 - Alanine (amino acid) H2N C H COOH Enantiomers are different compounds: Same boiling point, melting point, density Rotate plane polarized light in opposite directions (polarimetry) Different interaction with other chiral molecules Enzymes Receptor CHIRALITY AND CHIRAL COMPOUNDS What is chirality? Chirality (cheir, Greek for hand). The property of nonsuperimposability of an object on its mirror image is called chirality. If a molecule is not superimposable on its mirror image, it is chiral. If it is superimposable on its mirror image, it is achiral. Chirality • Carbons with four different groups attached to them are handed, or chiral. • Optical isomers or stereoisomers • If one stereoisomer is “right-handed,” its enantiomer is “left-handed.” CHIRAL COMPOUNDS Compounds which contain chiral carbon. Chiral carbon: It is an sp3-hybridized carbon atom with four different groups attached to it. Chiral compound exists in a pair of enantiomers. Chirality S-ibuprofen • Many pharmaceuticals are chiral. • Often only one enantiomer is clinically active. Enantiomers have identical physical and chemical properties except in two important respects: 1. They rotate the plane polarized light in opposite directions, however in equal amounts. The isomer that rotates the plane to the left (anticlockwise) is called the levo isomer and is designated (-) While the one that rotates the plane to the right (clockwise) is called the dextro isomer and designated (+). 2. They react at different rates with other chiral compounds. This is the reason that many compounds are biologically active while their enantiomers are not. They react at the same rates with achiral compounds. Stereochemistry Optical isomerism Determination of Number of Enantiomers [stereoisomers] 2n where n = number of chiral carbins n = zero 1 2 3 4 5 no possible stereoisomers 2 enantiomers are possible 4~ ~ ~ ~ ~ ~ ~ 8~ ~ ~ ~ ~ ~ 16 ~ ~ ~ ~ ~ ~ 32 ~ ~ ~ ~ ~ ~ Summary Stereoisomers: isomers that have same formula and connectivity but differ in the position of the atoms in space. They possess one or more stereocenters. Stereocenter: a carbon atom bearing 4 different atoms or group of atoms. Chiral: any molecule that is nonsuperposable with its mirror image. Enantiomers: stereoisomers that are non superposable mirror images. Optically Active: the ability of some compounds to rotate plane polarized light. TASK 1) 2) 3) 4) Which of the following molecules are optically active? propan-2-ol 2-chlorobutane 1-chlorobutane 3-methylhexane 5) 6) 7) 8) butanone 2-methylbutanoic acid butan-2-ol 1-chloro-3-methylpentane