Naming Covalent compounds power Point Presentation
advertisement

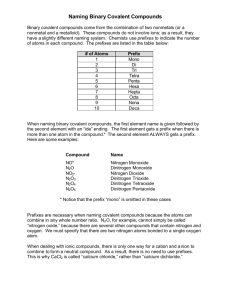
Naming Covalent Compounds Day 2 Nomenclature Bell Ringer Complete the chart for the following compounds using the rules for Naming Type I and Type II Compounds. Formula Type Compound Name Calcium Bromide PbO Iron (II) Oxide SnO BaCl2 Review - What is a covalent compound? Covalent compound: forms between 2 nonmetals Remember when Hydrogen bonds with a nonmetal this is a covalent compound too! Example: CO2, H2O, etc Naming Covalent Compounds Type III 1. The first element in the compound is named first using the name of the element 2. The second element is named as though it were an anion (obviously it isn’t really an anion since it is covalent); so the root word + the suffix “ide” 3. Prefixes are used to denote the numbers of each atom present (exception: the prefix “mono” is never used with the first element); CO is called carbon monoxide, not monocarbon monoxide Ex: Co2 would be named: Carbon Dioxide List of Prefixes (you will have a copy at your table) Number of Atoms Prefix 1 Mono- 2 Di- 3 Tri- 4 Tetra- 5 Penta- 6 Hexa- 7 Hepta- 8 Octa- 9 Nona- 10 Deca- Table Group Problems: 1. 2. 3. 4. 5. 6. N2O NO2 N2O3 N2O4 Phosphorous Pentachloride (write the formula) SO2 Table Group Answers: 1. Dinitrogen Oxide 2. Nitrogen dioxide 3. Dinitrogen trioxide 4. Dinitrogen Tetraoxide 5. PCl5 6. Sulfur Dioxide Naming Acids What ion do all acids have in common???? Has an H+ in its formula!! Typically the hydrogen cation is written first! Example of a classic acid: HCl for hydrochloric acid You are now going to learn the rules for naming acids Rules for Naming Acids: Rules for Naming Acids that Do Not Contain Oxygen in the Anion: Since all these acids have the same cation, H+, we don't need to name the cation. The acid name comes from the root name of the anion name. The prefix hydro- and the suffix -ic are then added to the root name of the anion. Example: HCl would be named as: Hydrochloric Acid Table Group Problems: HF 2. Hydrobromic Acid 3. HCl 4. Hydroiodic Acid 1. Answers to Table Group Problems: 1. Hydrofluoric Acid 2. HBr 3. Hydrochloric Acid 4. HI Independent Practice: Using your notes do more practice problems naming covalent compounds and acids (that do not contain oxygen) We will go over the answers at the end Make sure you do EVERY problem so you are ready to get that 100% on the exit slip… Exit Slip 1. What makes up a covalent compound? 2. Your classmate was given the compound CO and named the it Monocarbon Monoxide. Explain why his answer is correct or incorrect. If incorrect- write the correct nomenclature 3. Name the following: HF 4. Name the following: NO 5. Provide the chemical formula for: Sulfur Hexafluoride