Webquest-BONDING COMPOUND FORMATION
advertisement

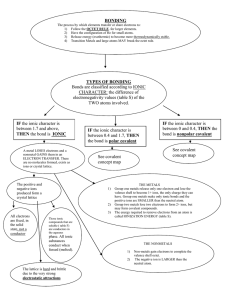
WEBQUEST—BONDING RESEARCH Research the answers to the following questions: 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. 12. 13. 14. 15. 16. 17. 18. 19. 20. 21. 22. 23. 24. 25. Ionic bonding occurs between what 2 types of ions? Nonmetals ______electrons and metals ________electrons. When the sodium ion bonds with the chlorine ion what neutral compound is formed? What element is always written first in an ionic compound? The nonmetal in an ionic compound is changed to end in the suffix -___________. What is a polyatomic ion? What are the representative elements? Give the charge for 1A, 2A, and 3A. What family is inert? What does this mean? List 2 descriptions of positive ions. List 2 descriptions of negative ions What holds ionic compounds together? What do metals do with their valence electrons? What do nonmetals do with their valence electrons? What is meant by simple binary compounds? Name MgS _________________ HCl _______________ SrO _______________________ Which metals have more than 1 charge? What’s the charge of the lanthanide and actinide series elements? Covalent formulas all end in -_________. What’s the difference between ionic & covalent bonds? What is a polyatomic ion? Give the 5 examples of polyatomic ions. Discuss the difference in the melting & boiling points of ionic vs. covalent compounds. Discuss the difference in the solubilities of ionic vs. covalent compounds.