Chemistry Scavenger Hunt
advertisement

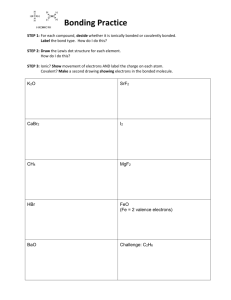
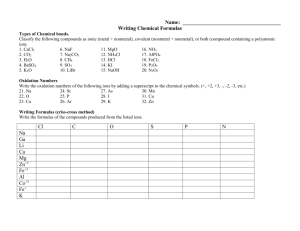
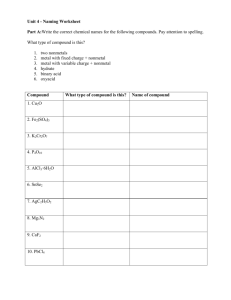
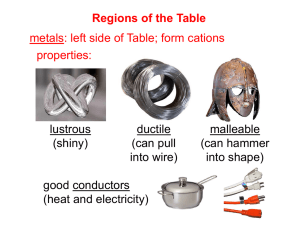
Chemistry Scavenger Hunt An Extra Credit Opportunity You are to find examples of the following. All samples should be in an appropriate container (Ziploc bag) and each container should have attached to it a 3 inch by 5 inch index card with the following information: Your name, class period, question #, answer to questions asked about the substance or required calculations. No substance can be used for more than one item on the list. The entire collection should be in a box. The box should be labeled with your name and class period. Use tiny amounts of each substance. Do not include whole bottles or packages of anything. You must include the information asked for in each question. No work, no credit, no kidding!!! 2 points per each correctly done item. No partial credit. 34 points maximum. 1. A chemistry cartoon cut from a newspaper or magazine. Photocopies are not acceptable. On the back of the note card include a brief statement about the concept that the cartoon is satirizing. 2. Iron (III) oxide. Common name and chemical name required. Balanced chemical equation for the production of the substance. 3. An ionically bonded substance and a covalently bonded substance. Label each with the substance it represents. Chemical formulas, common names and chemical names required. 4. Dilute acetic acid. Common name and chemical formula required. 5. SiO2; Common name and chemical name required. 6. A metalloid. Common name, chemical name, chemical formula required. 7. Something containing titanium (IV) oxide. Include formula on notecard. 8. An alkaline earth hydroxide. Chemical formula, common name and chemical name required. 9. A compound composed of an alkali metal and a halogen. Common name, chemical name, and chemical formula required. 10. Something containing a transition element from period 4. Identify the transition element on the back of the note card. 11. A nonmetal. Define nonmetal. Common name and chemical formula required. 12. An electrolyte. Define electrolyte. Common name, chemical name, chemical formula required. 13. An aqueous solution of a nonelectrolyte. Identify the solute and solvent by common name. Explain completely how you know it is a nonelectroyte solution. 14. A molecule with bonding that follows the octet rule. Write the molecular formula. Draw the Lewis Structure. Include common name and chemical name. 15. A hydrated crystal. Chemical formula, common name and chemical name required. 16. An oxide of an element. Common name, chemical name, chemical formula required. 17. The salt produced when magnesium hydroxide reacts with sulfuric acid. Balanced equation, chemical formula, chemical name, and common name required.