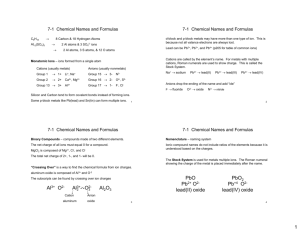
Naming Chemicals
advertisement

Name: _____________________________________ Writing Chemical Formulas Types of Chemical bonds. Classify the following compounds as ionic (metal + nonmetal), covalent (nonmetal + nonmetal), or both (compound containing a polyatomic ion). 1. CaCl2 6. NaF 11. MgO 16. NO2 2. CO2 7. Na2CO3 12. NH4Cl 17. AlPO4 3. H2O 8. CH4 13. HCl 18. FeCl3 4. BaSO4 9. SO3 14. KI 19. P2O5 5. K2O 10. LiBr 15. NaOH 20. N2O3 Oxidation Numbers Write the oxidation numbers of the following ions by adding a superscript to the chemical symbols. (+, +2, +3, -, -2, -3, etc.) 21. Na 24. Sr 27. As 30. Mn 22. O 25. P 28. I 31. Cu 23. Ca 26. Ar 29. K 32. Zn Writing Formulas (criss-cross method) Write the formulas of the compounds produced from the listed ions. Cl Na Ga Li Ca Mg Zn+2 Fe+3 Al Co+2 Fe+ K C O S P N Writing Formulas (criss-cross method) Write the formulas of the compounds produced from the listed ions. Cl- CO3-2 OH- SO4-2 PO4-3 NO3- Na+ NH4+ K+ Ca+2 Mg+2 Zn+2 Fe+3 Al+3 Co+3 Fe+2 H+ Naming Chemicals If you are given the formula, write the name of the chemical. If you are given the name, write the formula. You may use the rules of naming sheet. Ionic: 1) KCl 2) MgCl2 3) Lithium Bromide 4) KI 5) Calcium Chloride 6) NaF 7) Potassium Oxide 8) Magnesium Oxide Covalent 9) Carbon dioxide 10) CO 11) Sulfur dioxide 12) SO3 13) Dinitrogen pentoxide 14) P2O5 15) Carbon tetrachloride 16) OF2 Polyatomic Ions 17) Ammonia 18) Mg(NO3)2 19) Aluminum Phosphate 20) BaSO4 21) Na2CO3 22) Sodium Hydroxide 23) Be(NO3)2 24) (NH4)3PO4 25) Sodium Chlorate Mixed – you first have to determine what type of compound you have then use the correct rules to answer the question. 26) Iron II Chloride 27) Al2(CO3)3 28) Diphosphorus monoxide 29) N3O4 30) NaBr 31) Na2S 32) AsCl3 33) Sodium Nitrate 34) Sulfur monochloride 35) Zinc III oxide